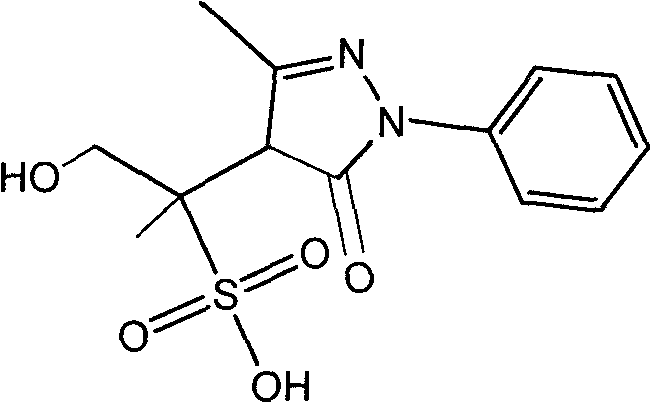
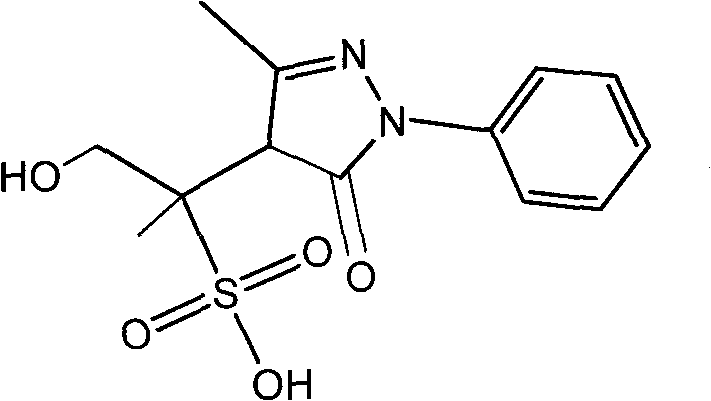
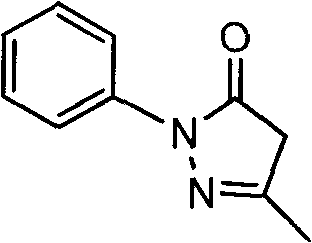
Pyrazolines compound as well as application and preparation method thereof
A compound, sodium metabisulfite technology, applied in the direction of organic chemistry, etc., can solve problems such as drug quality decline, and achieve the effect of simple method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 4g of Edaravone, 5g of sodium metabisulfite, 50g of 1,2-propanediol, and 50mL of water, respectively, and place them in an open container.
[0030] The suspension was placed in an oil bath at 50°C for heating and stirring, and was taken out after 96 hours, allowed to cool to room temperature, filtered, and the filtrate was set aside.
[0031] The above-mentioned compound A solution was separated by reverse-phase medium-pressure liquid chromatography, and 103 mg of compound A was prepared, with a yield of 1.4%.
Embodiment 2
[0033] Weigh 100 mg of Edaravone, 50 mg of sodium metabisulfite, 500 mg of 1,2-propanediol, and 1 mL of DMSO, respectively, and place them in an open container.
[0034] The solution was placed in an oil bath at 40°C for heating and stirring. After 168 hours, it was taken out, allowed to cool to room temperature, filtered, and the filtrate was set aside.
[0035] The above compound A solution was separated by reverse-phase high performance liquid chromatography, and 5 mg of compound A was prepared with a yield of 2.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com