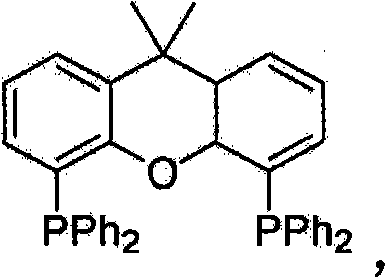
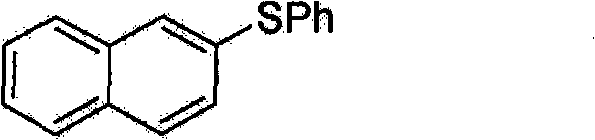Methods for the synthesis of organic sulfides by using sulfides and organic sulfur-indium complexes
A technology of organic sulfur and complexes, applied in the field of synthesis of carbon-sulfur bonds, which can solve problems such as long reaction time and excess catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of 2-phenyl naphthyl sulfide
[0045] Formula 5
[0046]
[0047] A solution of palladium acetate (4.5 mg, 0.02 mmol) and Xantphos (12.7 mg, 0.022 mmol) in DMF (1 mL) was stirred for 5 minutes under nitrogen atmosphere. To this solution was added 2-bromonaphthalene (103.5 mg, 0.5 mmol) dissolved in 0.5 mL of DMF, and the mixture was stirred at room temperature for 10 min. To the reaction mixture was added In(SPh) in DMF (1 mL) 3 (74mg, 0.167mmol) and diisopropylethylamine (65mg, 0.5mmol). The reaction mixture was stirred at 100°C for 2 hours. The solution was cooled to room temperature, and 1 mL of hydrochloric acid (5% aqueous solution) was added thereto to terminate the reaction. The crude product was extracted with diethyl ether (15 mL, 3 times), and washed successively with 10 mL of water, saturated NaHCO 3 solution (10 mL) and saturated NaCl solution (20 mL). Extract organic compounds with anhydrous MgSO 4 Dry and filter. Af...
Embodiment 2
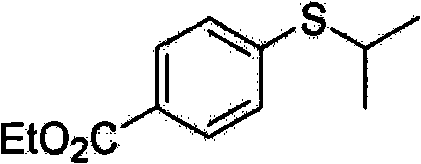
[0049] Embodiment 2: the preparation of ethyl 3-isopropylthiobenzoate
[0050] Formula 6
[0051]
[0052] A solution of palladium acetate (4.5 mg, 0.02 mmol) and Xantphos (12.7 mg, 0.022 mmol) in DMF (1 mL) was stirred for 5 minutes under nitrogen atmosphere. To this solution was added ethyl 3-bromobenzoate (114.5 mg, 0.5 mmol) dissolved in 0.5 mL of DMF, and the reaction mixture was stirred at room temperature for 10 minutes. To the reaction mixture was added In(SPr i ) 3 (57mg, 0.168mmol) and diisopropylethylamine (65mg, 0.5mmol). The reaction mixture was stirred at 100°C for 2 hours. The solution was cooled to room temperature, and 1 mL of hydrochloric acid (5% aqueous solution) was added thereto to terminate the reaction. The crude product was extracted with diethyl ether (15 mL, 3 times), and washed successively with 10 mL of water, saturated NaHCO 3 solution (10 mL) and saturated NaCl solution (20 mL). Extract the organic compounds with anhydrous MgSO 4 Dry a...
Embodiment 3
[0054] Embodiment 3: Preparation of 1-phenylnaphthyl sulfide
[0055] Formula 7
[0056]
[0057] A solution of palladium acetate (4.5 mg, 0.02 mmol) and Xantphos (12.7 mg, 0.022 mmol) in DMF (1 mL) was stirred for 5 minutes under nitrogen atmosphere. To this solution was added 1-naphthalene triflate (138.1 mg, 0.5 mmol) dissolved in DMF (0.5 mL), and the reaction mixture was stirred at room temperature for 10 minutes. To the reaction mixture was added In(SPh) in DMF (1 mL) 3 (74mg, 0.167mmol)) and diisopropylethylamine (65mg, 0.5mmol). The reaction mixture was maintained at 100°C for 2 hours. The solution was cooled to room temperature, and 1 mL of hydrochloric acid (5% aqueous solution) was added thereto to terminate the reaction. The crude product was extracted with diethyl ether (15 mL, 3 times), and washed successively with 10 mL of water, saturated NaHCO 3 solution (10 mL) and saturated NaCl solution (20 mL). Extract the organic compounds with anhydrous MgSO 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com