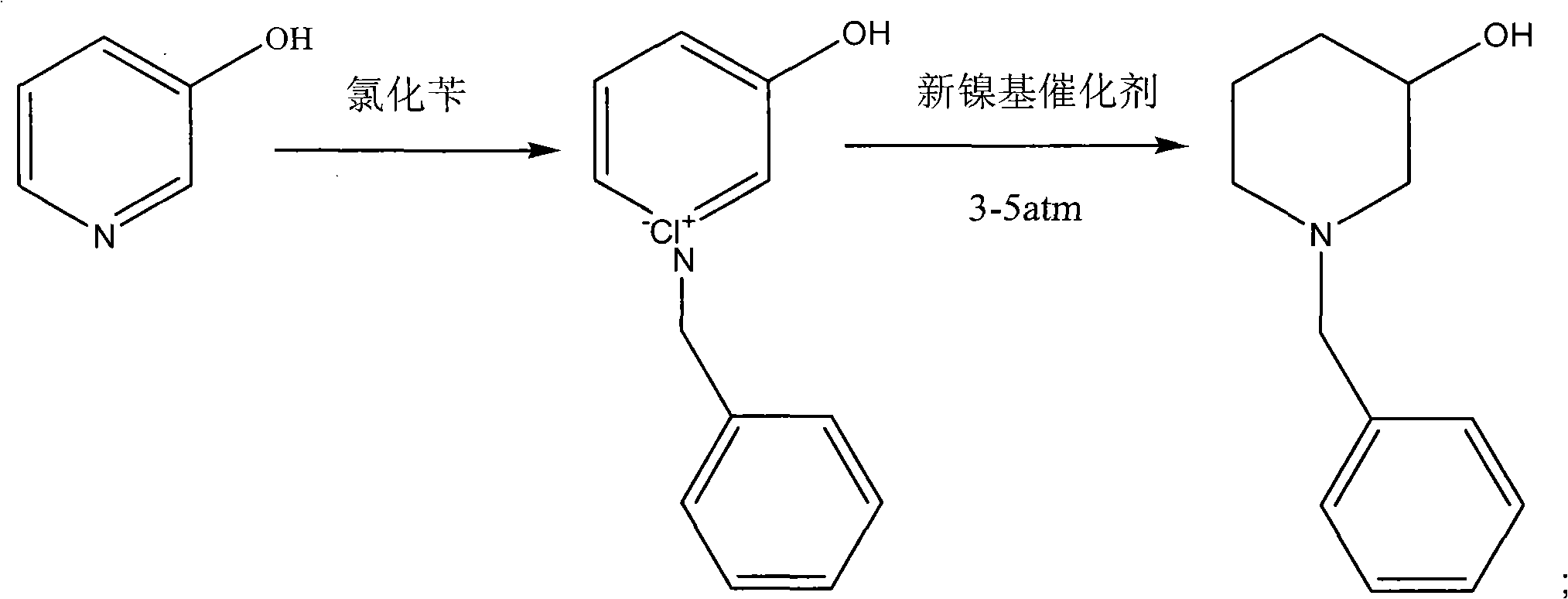
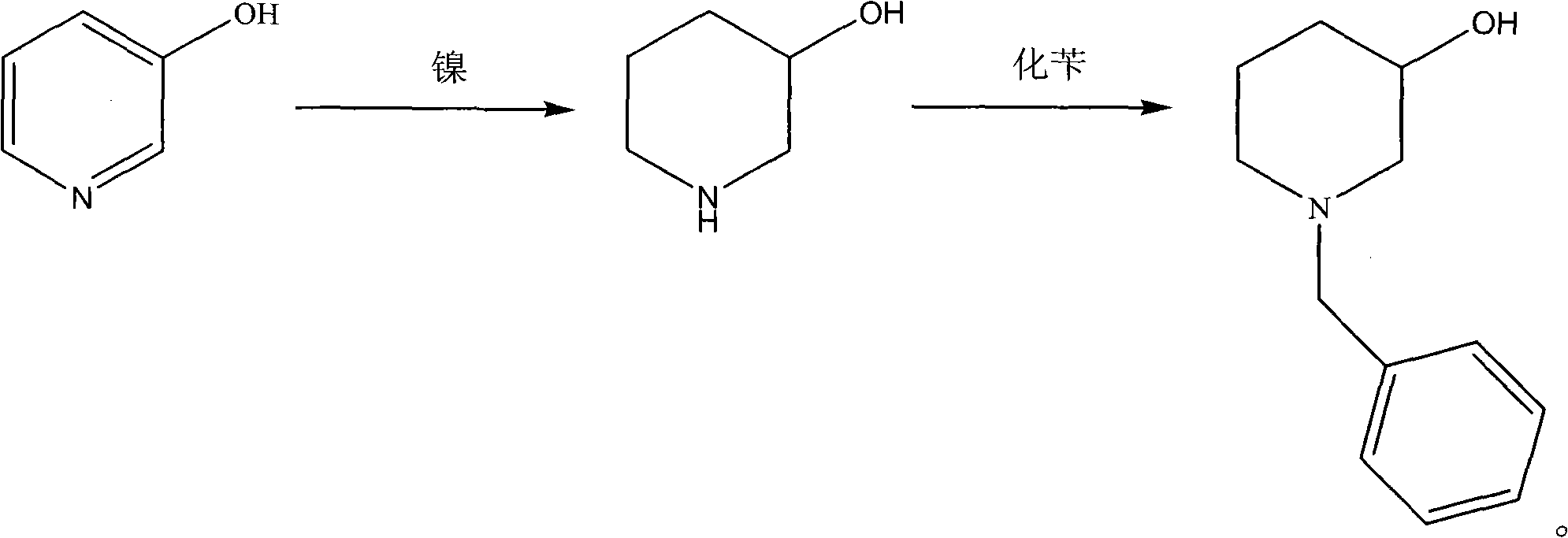
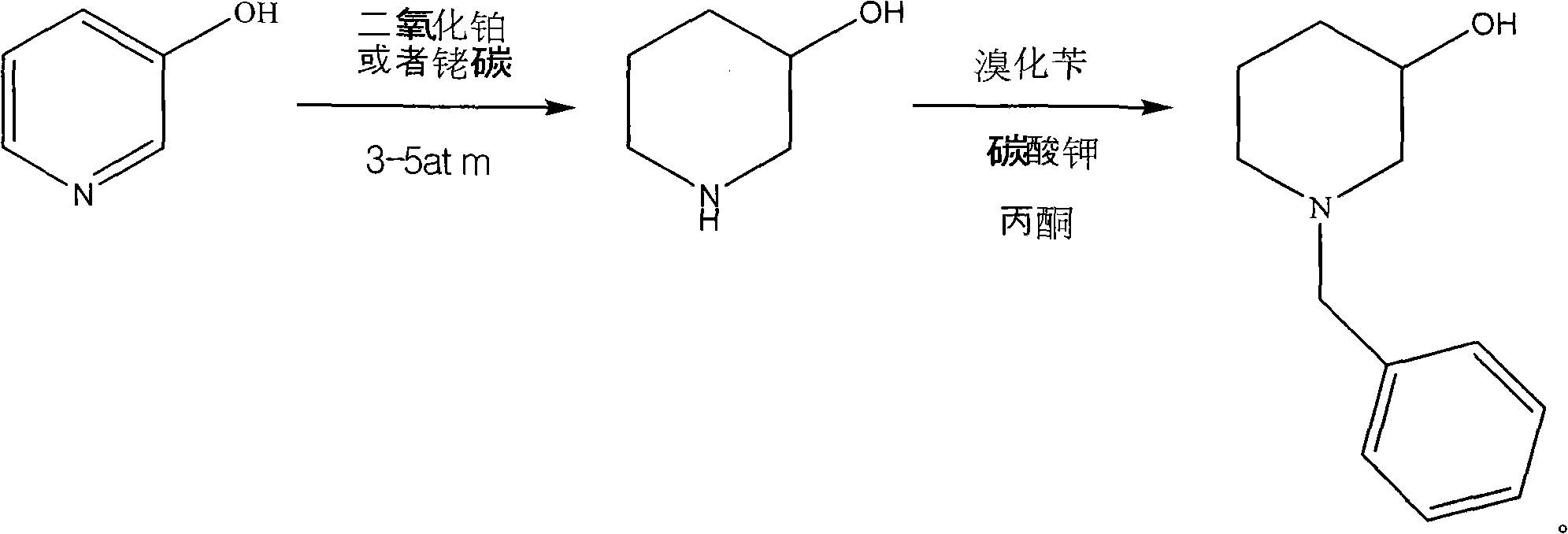
Novel synthesis process of N-benzyl-3-piperidinol, novel nickel-based catalyst and preparation method thereof
A technology of nickel-based catalyst and piperidinol, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the requirement of reducing hydrogen pressure, and the cost is difficult to reduce , high cost of noble metal catalysts, etc., to achieve the effects of lower pressure resistance requirements, lower production costs, and significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Prepare new nickel-based catalyst as follows:
[0048] (1) 700g of diatomaceous earth is added into a three-necked round bottom flask with a capacity of 20L;
[0049] (2) 1250ml of 95% ethanol, 290g of nickel acetate, and 84.25g of cobalt chloride were also added to the aforementioned three-necked round-bottomed flask, fully dissolved, and soaked overnight;
[0050] (3) Slowly add 80 g of sodium borohydride solution (0.2 mol / L) under vigorous stirring, and fully react until no bubbles are released;
[0051] (4) After completion of the reaction, add 4L of water and filter with suction to obtain about 850g of the new nickel-based catalyst (the active ingredient in which is about 150g);
[0052] (5) Wash the new nickel-based catalyst for 3 to 5 times, wash each time with 1000 ml of water, drain and store in 95% ethanol for later use.
Embodiment 2
[0054] Prepare N-benzyl-3-piperidinol as follows:
[0055] (1) Add 95g of 3-hydroxypiperidine and 500mL of toluene into a three-neck round bottom bottle equipped with mechanical stirring, thermometer, and constant pressure dropping funnel, and heat to 90°C;
[0056] (2) After the 3-hydroxypyridine is completely dissolved in toluene, 130 g of benzyl chloride is slowly added dropwise therein, and the dropwise addition is completed within 30 minutes;
[0057] (3) Keep at 90°C and react for 2 hours;
[0058] (4) stop heating, continue to stir and cool to room temperature, separate out a large amount of white solids;
[0059] (5) filter the separated white solid to obtain the quaternary ammonium salt of N-benzyl-3-hydroxypyridine, and wash the filter cake once with toluene 200mL;
[0060] (6) The quaternary ammonium salt of the washed N-benzyl-3-hydroxypyridine is added to the hydrogenation kettle, and 500 mL of absolute ethanol, 101 g of triethylamine, and 30 g of the new nickel...
Embodiment 3
[0070] Prepare N-benzyl-3-piperidinol according to the following method:
[0071] (1) Add 1.9Kg of 3-hydroxypiperidine and 10L of toluene into a 20L enamel reaction kettle equipped with mechanical stirring, enamel disk condenser, thermometer and metering pump, and heat to 110°C;
[0072] (2) After the 3-hydroxypyridine is completely dissolved in toluene, 2.6Kg of benzyl chloride is slowly added dropwise therein, and the dropwise addition is completed within 30 minutes;
[0073] (3) Keep at 110°C and react for 2 hours;
[0074] (4) stop heating, continue to stir and cool to room temperature, separate out a large amount of white solids;
[0075] (5) filter the separated white solid to obtain the quaternary ammonium salt of N-benzyl-3-hydroxypyridine, and wash the filter cake once with 4L of toluene;
[0076] (6) The quaternary ammonium salt of the washed N-benzyl-3-hydroxypyridine is added in a 20L hydrogenation kettle, and 600g of the new nickel-based catalyst of 10L of dehyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Relative density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com