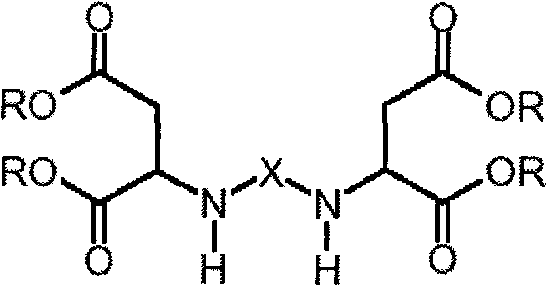
Method for preparing novel polyaspartic ester and application thereof
A new type of polyaspartic acid ester technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the physical properties of polyurea materials without mentioning tensile strength, elongation, and macromolecular primary Amine, gel time is not long enough, etc., to achieve the effect of changing low adhesion, excellent mechanical properties, and performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
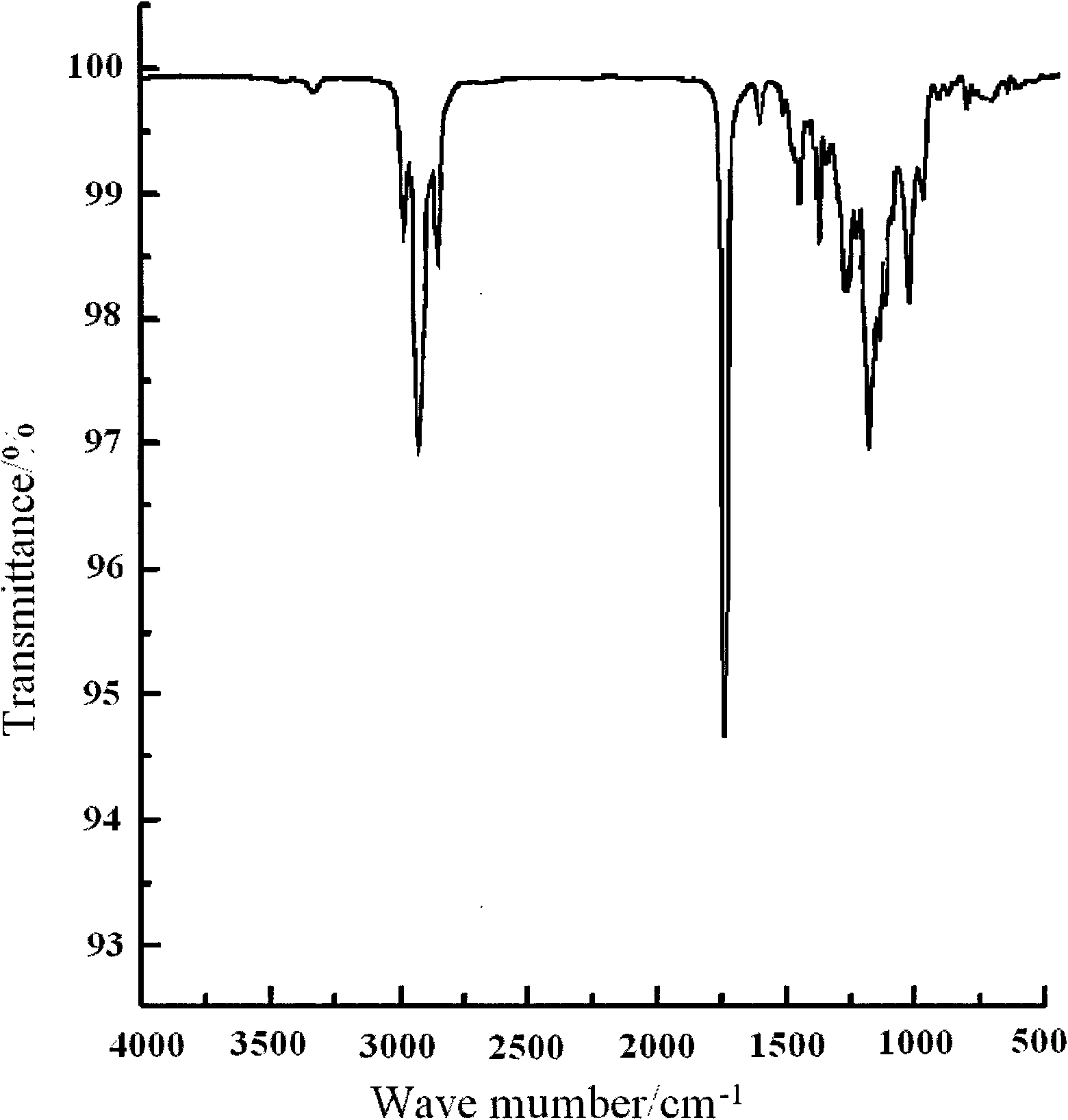
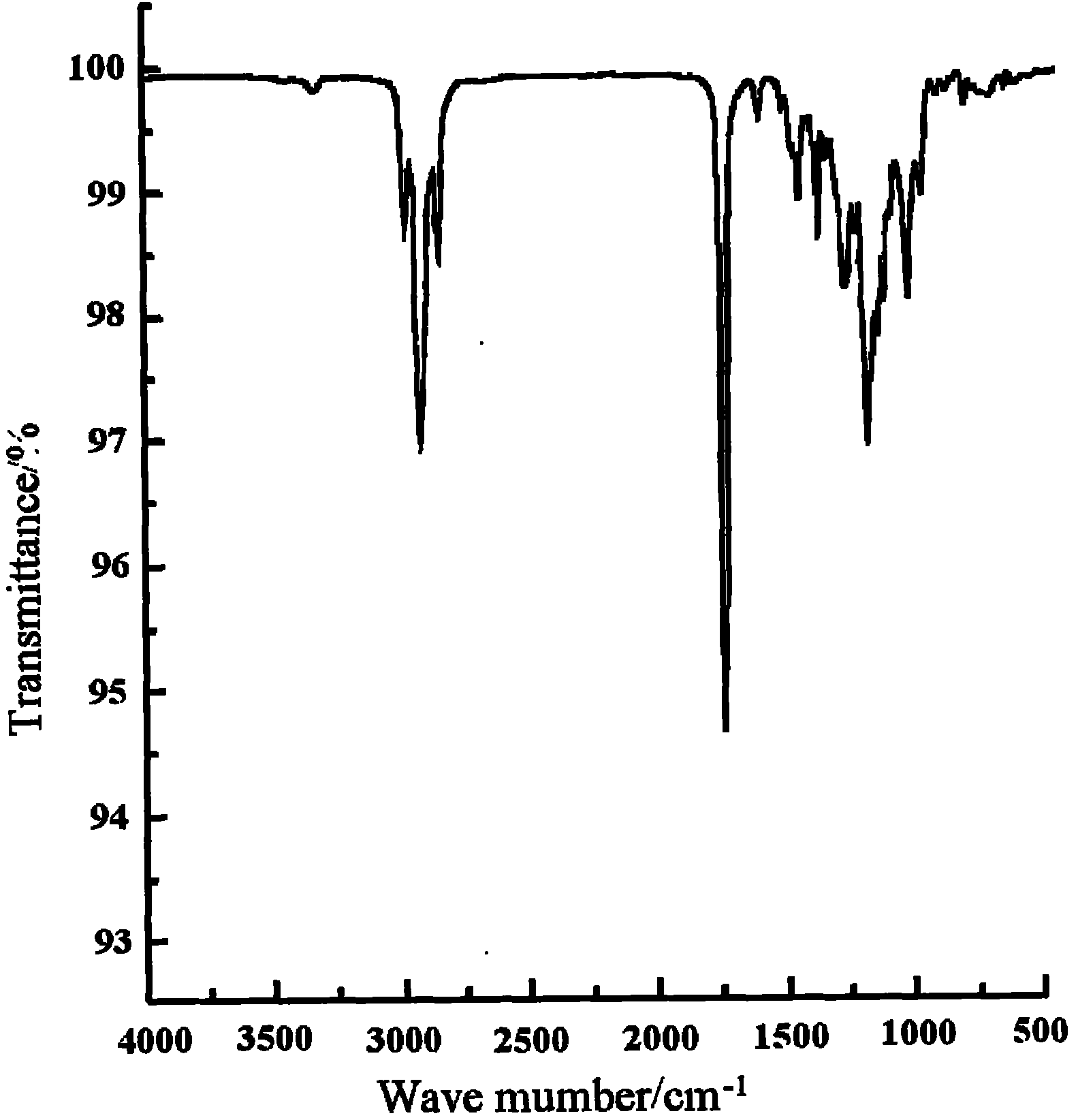
Image
Examples
Embodiment 1
[0032] 4,4'-Diaminocyclohexylmethane was vacuum-dried, diethyl maleate was distilled under reduced pressure, and epoxy resin was vacuum-dried.
[0033] The four-necked flask was equipped with a stirrer, heating unit, addition funnel and nitrogen inlet. 105 grams (0.5 equivalents) of 4,4'-diaminocyclohexylmethane was packed into a flask, and nitrogen protection was carried out. 172 g of diethyl maleate (1.0 equivalent) was added to the flask through an addition funnel within 1 hour, and the temperature was controlled not to exceed 60° C. during the addition. After the feeding was completed, the temperature was controlled at 80° C., and the reaction was carried out for 20 hours. At this point 95% of the 4,4'-diaminocyclohexylmethane was converted to polyaspartate. Add 18 grams (0.09 equivalents) of bisphenol A epoxy resin E-51, control the addition within 10 minutes, raise the temperature to 120 ° C for 2 hours, cool to room temperature, and obtain a transparent, light yellow ...
Embodiment 2
[0035] Polyetheramine D-230 is vacuum-dried, diethyl maleate is distilled under reduced pressure, and epoxy resin is vacuum-dried.
[0036] The four-necked flask was equipped with a stirrer, heating unit, addition funnel and nitrogen inlet. Put 115 grams (0.5 equivalent) of polyetheramine D-230 into the flask, and protect it with nitrogen. 172 g of diethyl maleate (1.0 equivalent) was added to the flask through an addition funnel within 1 hour, and the temperature was controlled not to exceed 60° C. during the addition. After the feeding was completed, the temperature was controlled at 80° C., and the reaction was carried out for 20 hours. At this point 94% of the polyetheramine D-230 had been converted to polyaspartate. Add 18 grams (0.09 equivalents) of bisphenol A epoxy resin E-51, control the addition within 10 minutes, raise the temperature to 120 ° C for 2 hours, cool to room temperature, and obtain a transparent, light yellow liquid, and the reaction is 100% complete....
Embodiment 3
[0038] Polyetheramine D-2000 is vacuum-dried, diethyl maleate is distilled under reduced pressure, and epoxy resin is vacuum-dried.
[0039] The four-necked flask was equipped with a stirrer, heating unit, addition funnel and nitrogen inlet. Put 1000 g (0.5 equivalent) of polyetheramine D-2000 into the flask, and protect it with nitrogen. 172 g of diethyl maleate (1.0 equivalent) was added to the flask through an addition funnel within 1 hour, and the temperature was controlled not to exceed 60° C. during the addition. After the feeding was completed, the temperature was controlled at 80° C., and the reaction was carried out for 20 hours. At this point 93% of the polyetheramine D-2000 was converted to polyaspartate. Add 18 grams (0.09 equivalents) of bisphenol A epoxy resin E-51, control the addition within 10 minutes, raise the temperature to 120° C. and keep for 2 hours, and cool to room temperature to obtain a transparent, yellow liquid, and the reaction is 100% complete....
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com