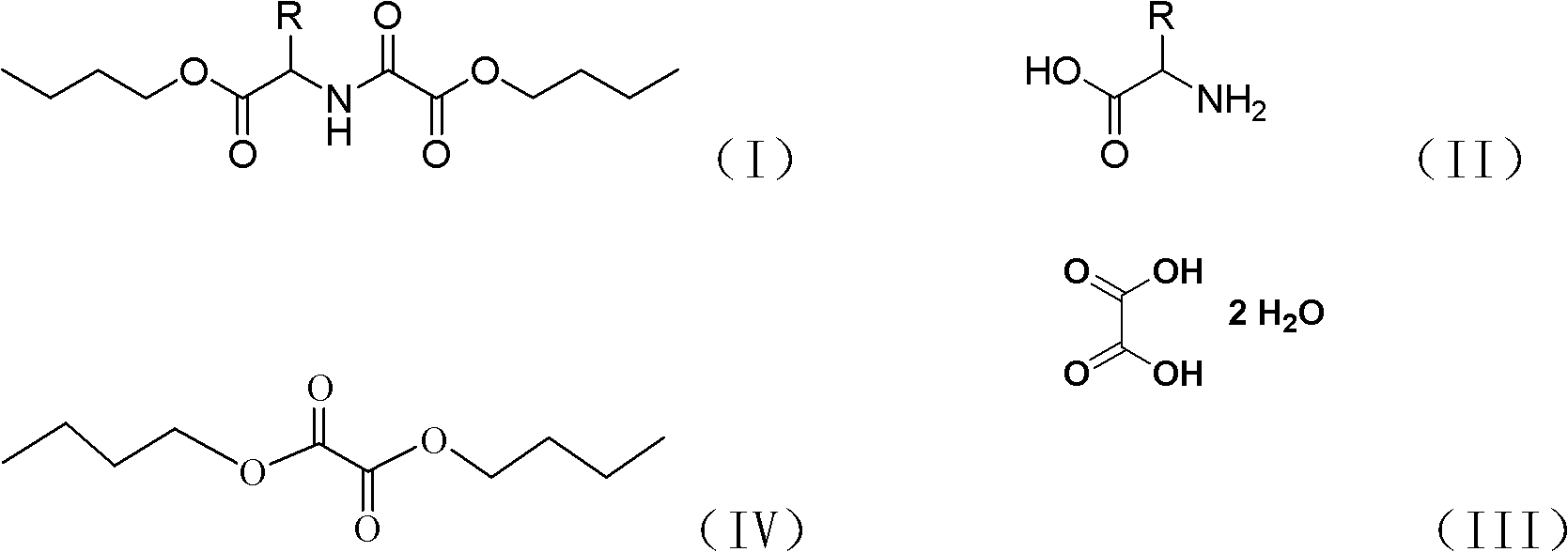
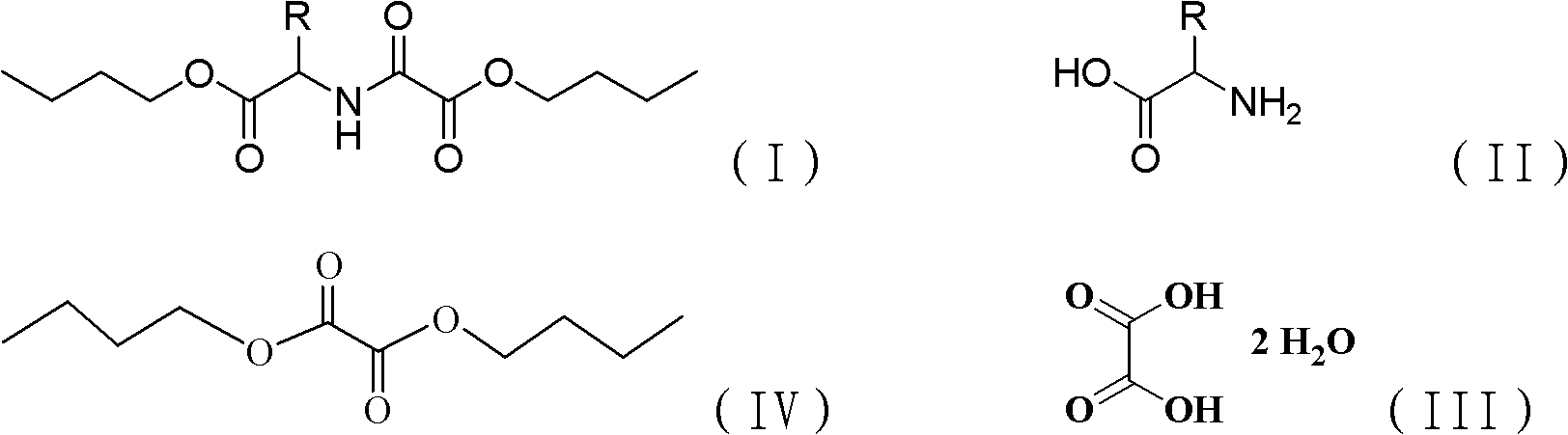
Chemical synthesis method of N-butoxyoxalyl amino acid butyl ester
A butyloxyoxalylamino acid butyl ester, chemical synthesis technology, applied in chemical instruments and methods, organic chemistry, carboxylic acid amide preparation, etc., to achieve the effects of simplifying the process, reducing corrosion, reducing equipment and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
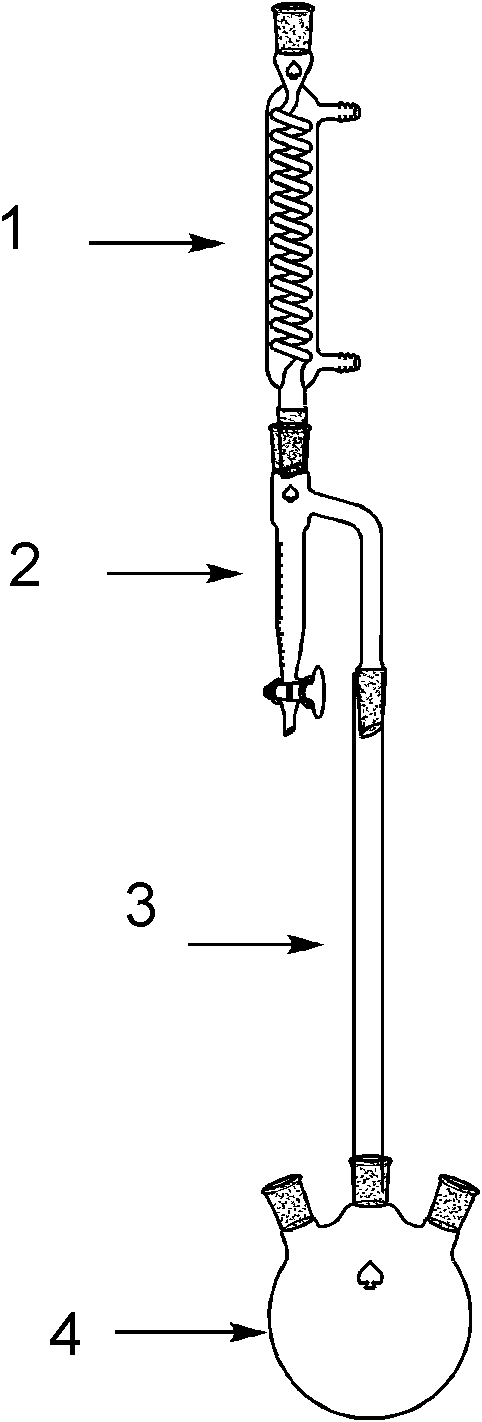
[0023] In a 1000ml four-necked flask equipped with a thermometer, stirring, rectifying column, water separator, and condenser, add 38g of glycine, 63g of oxalic acid (containing 2 crystal waters), and 400ml of n-butanol, heat to dissolve, and the solution is clarified 100 g of dibutyl oxalate was added, and it was made to react at 100 degreeC (internal temperature) for 35 hours. During the heating, rectification, dehydration and esterification process of the reaction liquid, the water layer is continuously separated from the water trap at the top of the rectification column, and the n-butanol layer flows into the column. After the reaction was complete, the reaction solution was washed with water, and n-butanol and dibutyl oxalate were recovered by distillation under reduced pressure. The rest was butyl N-butoxyoxalylglycinate to obtain 112 g of light yellow oil with a yield of 87%. 1 HNMR: 7.1 (sbr, 1H), 4.13 (t, 2H), 4.10 (t, 2H), 3.76 (s, 2H), 1.55-1.57 (m, 4H), 1.29-1.32 (...
Embodiment 2
[0025] The molar ratio of feeding is glycine: oxalic acid: dibutyl oxalate is 1:3:3, and the input glycine is 19g. Other conditions and preparation steps are the same as in Example 1. 55 g of butyl N-butoxyoxalylglycine was obtained, and the yield was 85%.
Embodiment 3
[0027] Feeding molar ratio is glycine: oxalic acid: dibutyl oxalate is 1: 2: 1, the glycine 38g that drops in, other conditions preparation steps are with embodiment 1. Obtain N-butoxyoxalylglycine butyl ester 111g, yield is 86% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com