Double metal cyanide-rare earth compound composite catalyst for CO2-epoxypropane copolymerization
A technology of double metal cyanide and rare earth complex, which is applied in the field of double metal cyanide-rare earth complex composite catalyst, can solve problems such as literature and patent reports that have not yet been studied, and achieve the effect of being beneficial to industrial development and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
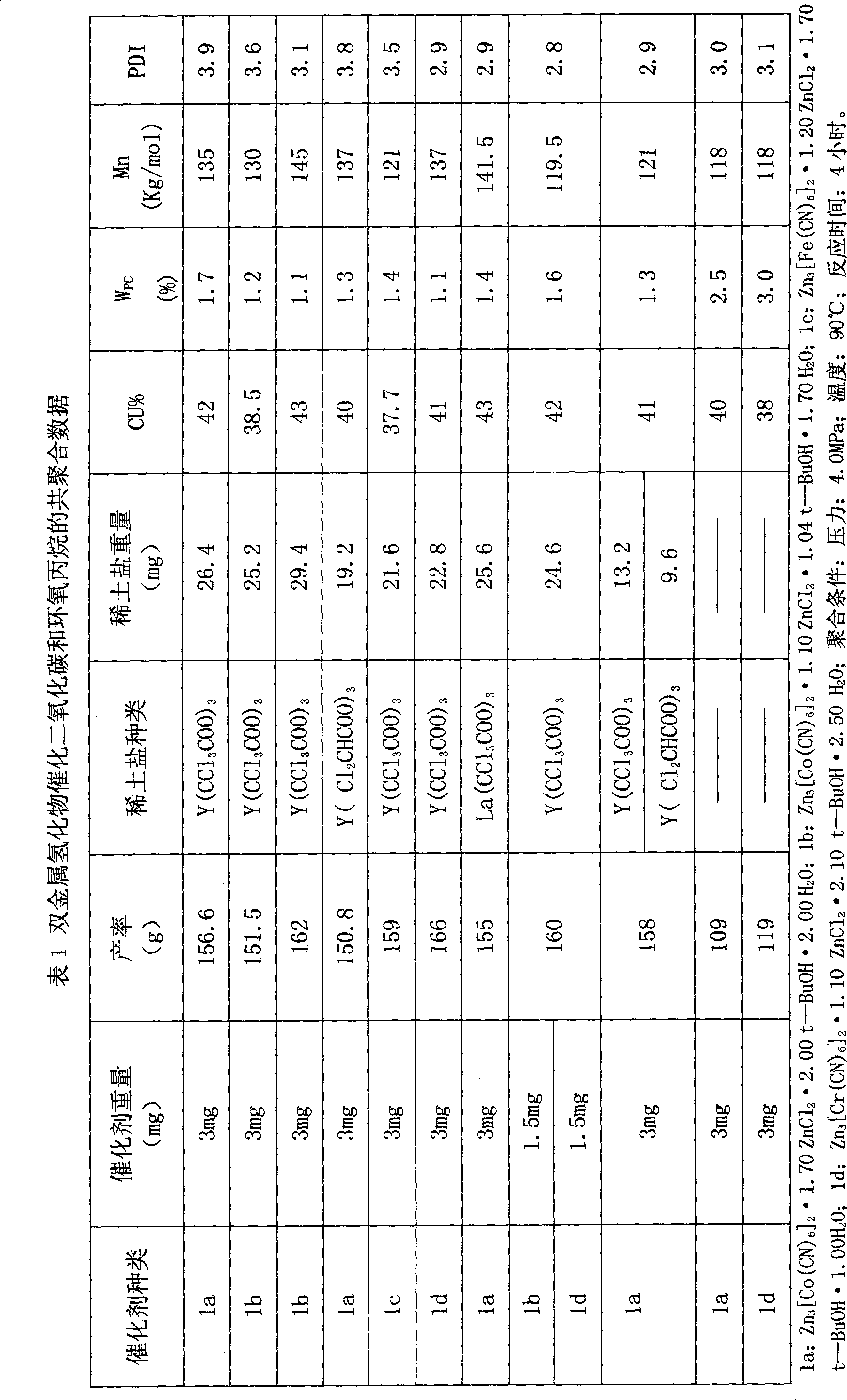
Image
Examples
preparation Embodiment 1
[0021] The preparation of preparation example 1 double metal cyanide:
[0022] 40g ZnCl 2 3.32g K 3 Co(CN) 6 and 50ml of deionized water, the dropwise addition process took 2 hours. After the dropwise addition was completed, the reaction was continued to stir at 500 rpm for 2 hours at 50°C. After the reaction, the reactant was centrifuged at 5,000rpm for 30 minutes to collect the precipitate, which was continuously washed with tert-butanol / water (V / Vml)=100 / 100, 120 / 80, 140 / 60, 160 / 40, The 180 / 20 mixed solution and 200ml tert-butanol were stirred at 60-100rpm to pulp and wash 6 times. The time for each pulping and washing was 40 minutes. After each pulping and washing, it was centrifuged at 5,000rpm for 30 minutes. The obtained final product was dried in a vacuum oven at 50°C to constant weight, and the product after constant weight was divided into ampoule bottles, and treated with Ar at 50°C to 20-40Pa for 48 hours, repeatedly filled with Ar for 24 times, and finally pro...
preparation Embodiment 2
[0023] The preparation of preparation embodiment 2 rare earth complexes:
[0024] Under stirring at 80 rpm, 25 g of diyttrium trioxide was added ten times, 2.5 g each time, into a three-necked flask with 48.9 g of trichloroacetic acid and 100 ml of deionized water, and the reaction temperature was controlled at 40-45°C. After all the diyttrium trioxide was added, stirring was continued at 40-45°C for 2.5 hours. The reactant is filtered to remove unreacted diyttrium trioxide, and the clear liquid is dehydrated at 40-45°C under reduced pressure at 1.13KPa until all the water is removed. The obtained white yttrium trichloroacetate complex powder was divided into ampoules, treated with vacuum at 80°C to 20-40Pa and filled with Ar for 80 hours. After filling with Ar 40 times, it was sealed and stored under the protection of Ar.
preparation Embodiment 3
[0025] The preparation of preparation embodiment 3 rare earth complexes:
[0026] Under stirring at 80rpm, 37g of diyttrium trioxide was added to a three-necked flask with 38.7g of dichloroacetic acid and 100ml of deionized water in ten times, 3.7g each time, and the reaction temperature was controlled at 40-45°C. After all the diyttrium trioxide was added, stirring was continued at 40-45°C for 3 hours. The reactants were filtered to remove unreacted diyttrium trioxide, and the clear liquid was dehydrated under reduced pressure at 40-45°C and 1.13KPa until all the water was removed. The obtained white yttrium dichloroacetate complex powder is divided into ampoule bottles, vacuumed at 80°C to 20-40Pa and filled with N 2 Treat for 80 hours, charge N 2 After 40 times, at N 2 Store under airtight protection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com