Hymecromone microemulsion medicament and preparation method thereof
A hymecoumarin and microemulsion technology, which is applied in the direction of drug combination, anti-infective drug, emulsion delivery, etc., can solve the problems of unreachable therapeutic dose, limited clinical application, low solubility, etc., and achieve significant acaricidal effect, Enhanced drug efficacy and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
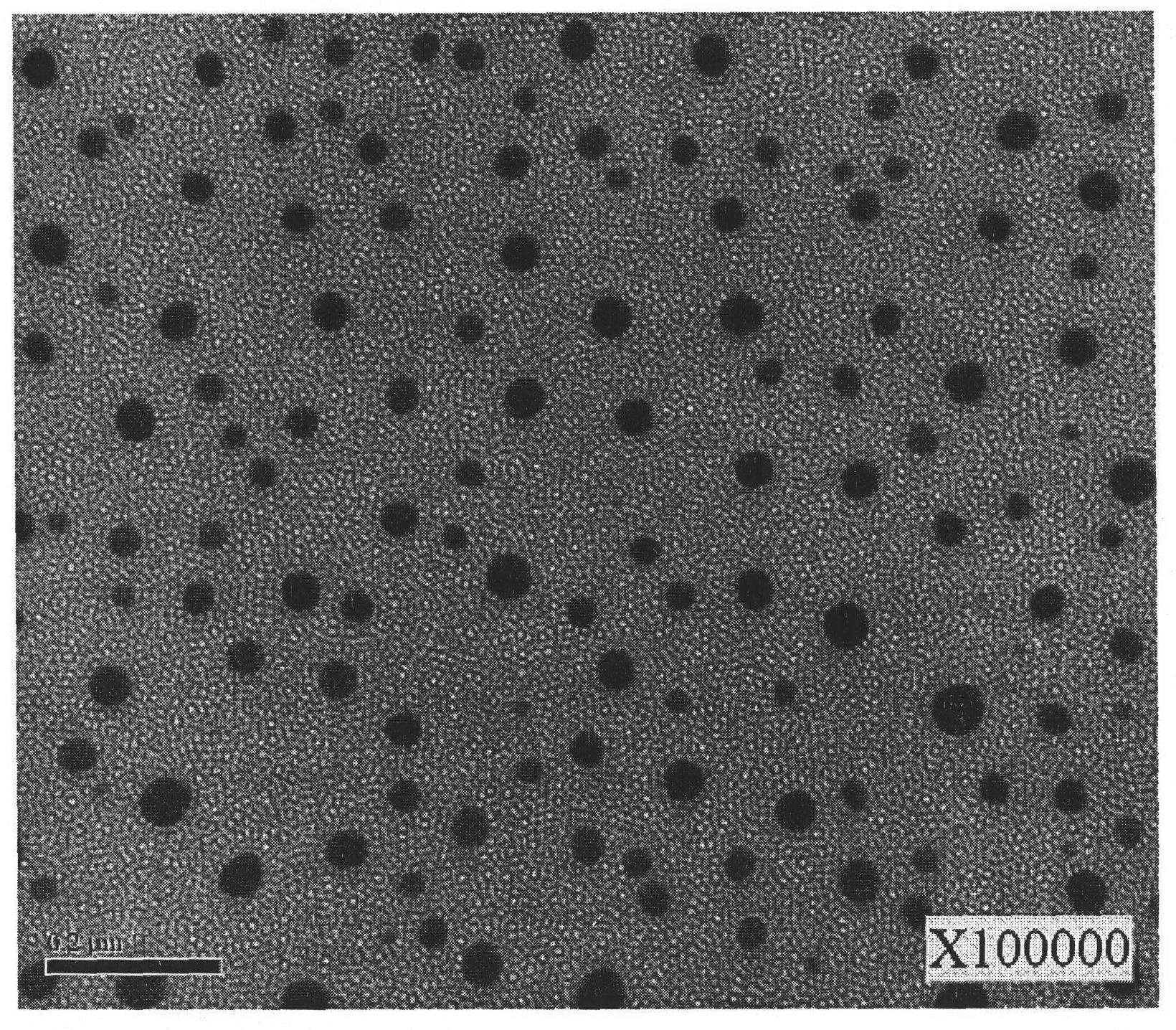
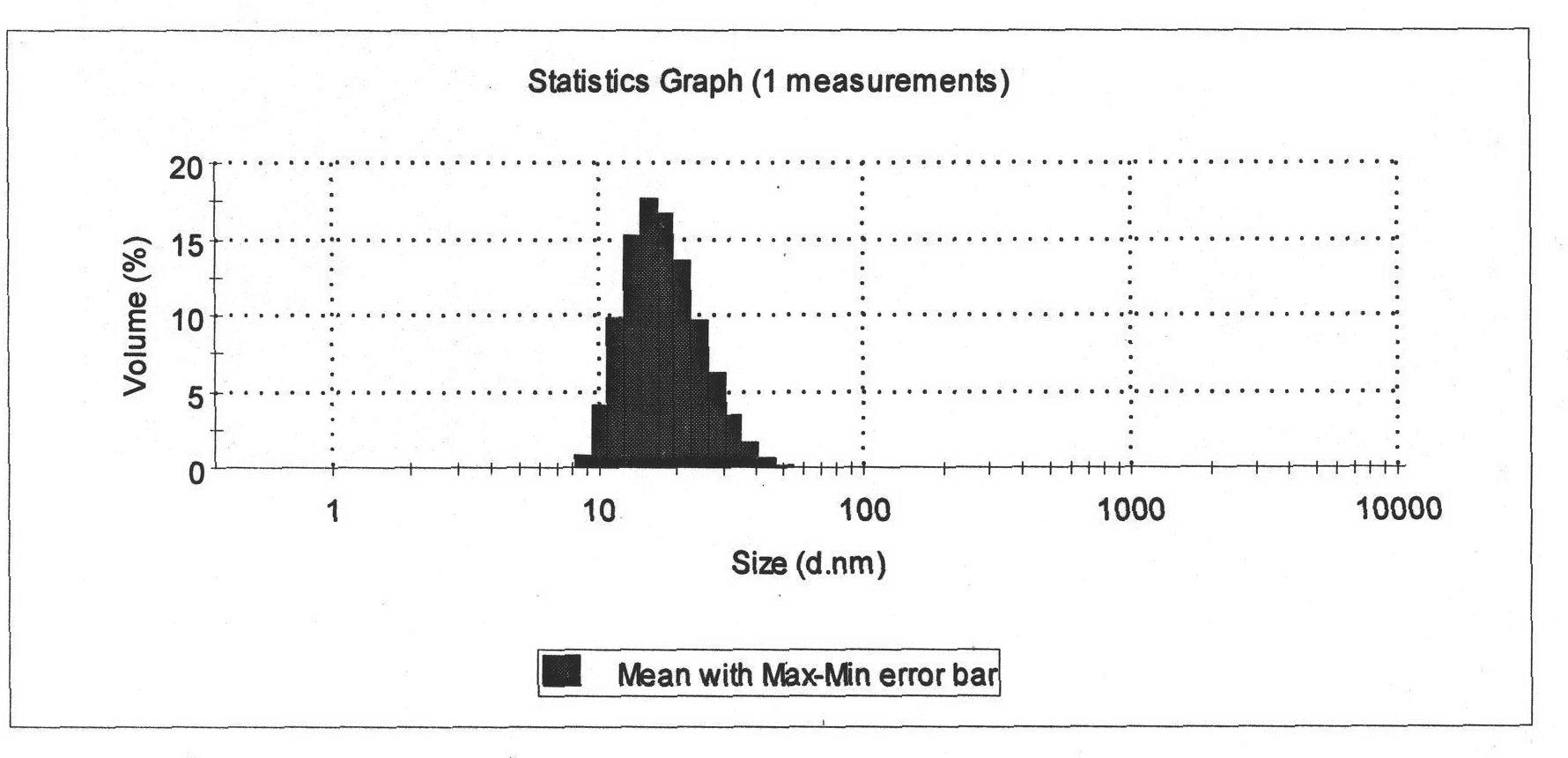
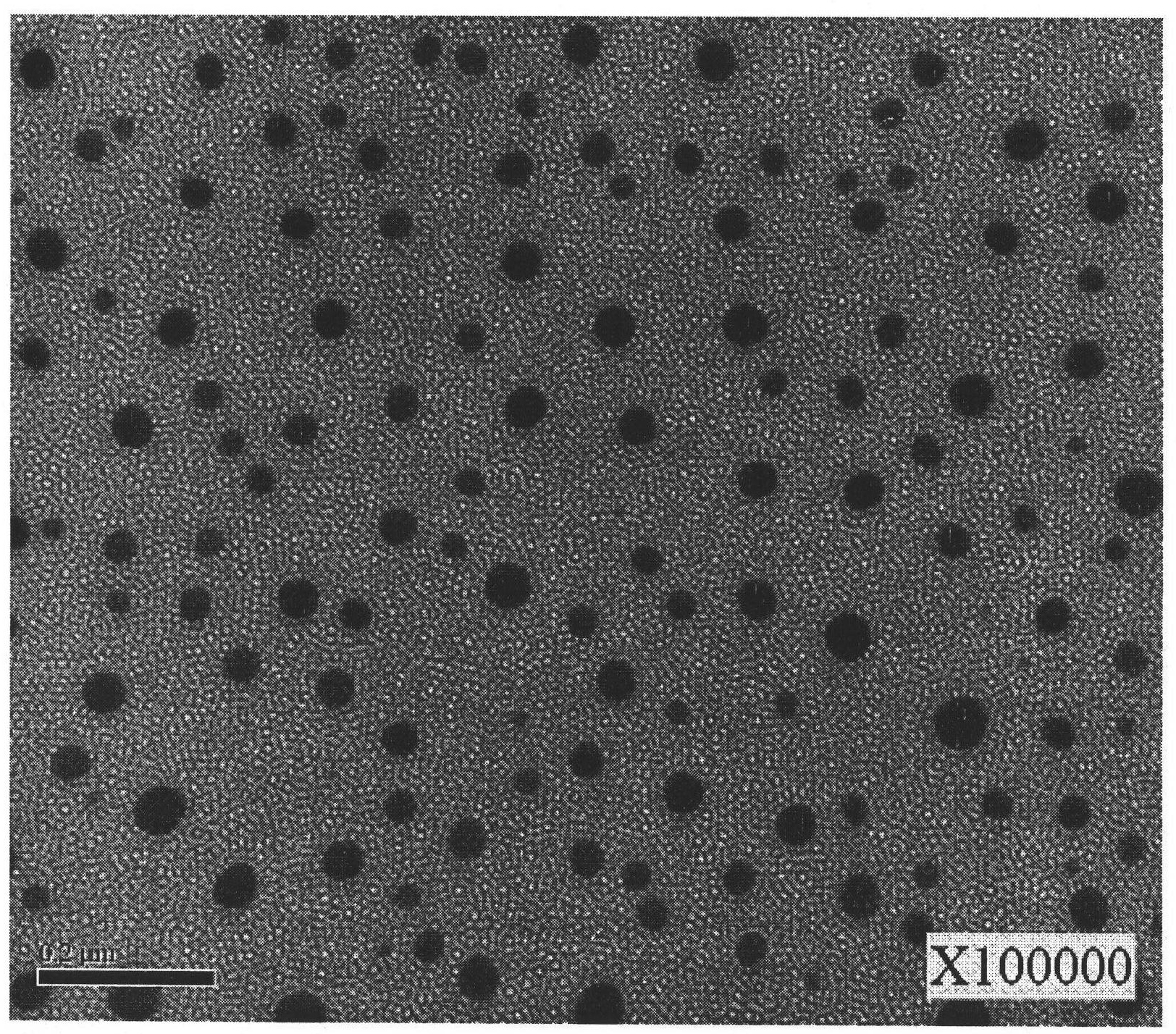
Image
Examples
Embodiment 1
[0047] Example 1 Prepared a hymecoumarin microemulsion with a drug concentration of 0.1%.
[0048] Hydroxymecoumarin
0.1g
IPM
5.0g
Tween80
27.0g
PEG400
18.0g
3.0g
46.9g
[0049] Preparation:
[0050] (1) Weigh 0.1 g of hymecoumarin, 5.0 g of IPM, 27.0 g of Tween800, 18.0 g of PEG400, 3.0 g of dimethyl sulfoxide, and 46.9 g of distilled water, and set aside.
[0051] (2) Mix hymecoumarin with dimethyl sulfoxide to dissolve, add IPM, Tween80, PEG400 and mix until completely mixed; slowly add distilled water dropwise, and keep stirring while adding until a clear and transparent system, and then drop distilled water to a sufficient amount to obtain the hymecoumarin microemulsion drug with a drug concentration of 0.1% of the present invention.
Embodiment 2
[0052] Example 2 The hymecoumarin microemulsion with a drug concentration of 0.3% was prepared.
[0053] Hydroxymecoumarin
0.3g
4.0g
EL-35
24.0g
16.0g
5.0g
distilled water
50.7g
[0054] Preparation:
[0055] (1) Weigh 0.3 g of hymecoumarin, 4.0 g of oleic acid, 4.0 g of EL352, 16.0 g of ethanol, 5.0 g of azone, and 50.7 g of distilled water, and set aside.
[0056] (2) Mix and stir the mixture of hydroxymecoumarol, oleic acid, EL35, ethanol and azone until the hydroxymecoumarin is completely dissolved; slowly add distilled water dropwise, and keep stirring while dropping until a clear and transparent system, and then drop distilled water to a sufficient amount to obtain the hymecoumarin microemulsion drug with a drug concentration of 0.2% of the present invention.
Embodiment 3
[0057] Example 3 The hymecoumarin microemulsion with a drug concentration of 0.3% was prepared.
[0058] Hydroxymecoumarin
[0059] Preparation method: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com