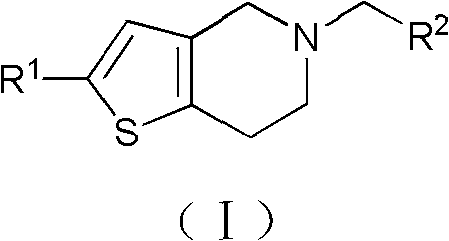
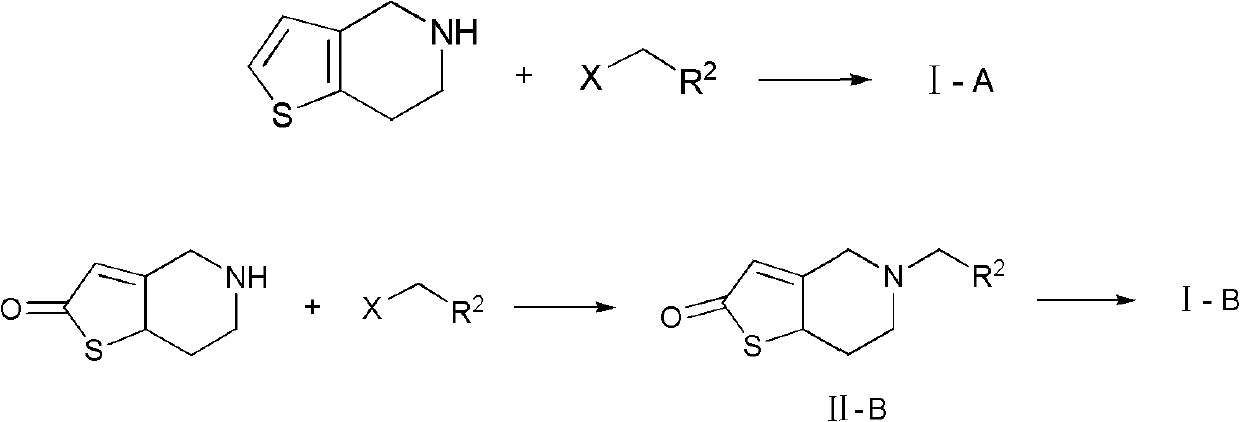
Nitrogen-containing heterocyclic thienopyridine compounds and preparation method and application thereof
A compound, pyridine technology, applied in the field of medicine, can solve problems such as poor selectivity, low specificity, multi-drug resistance of tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
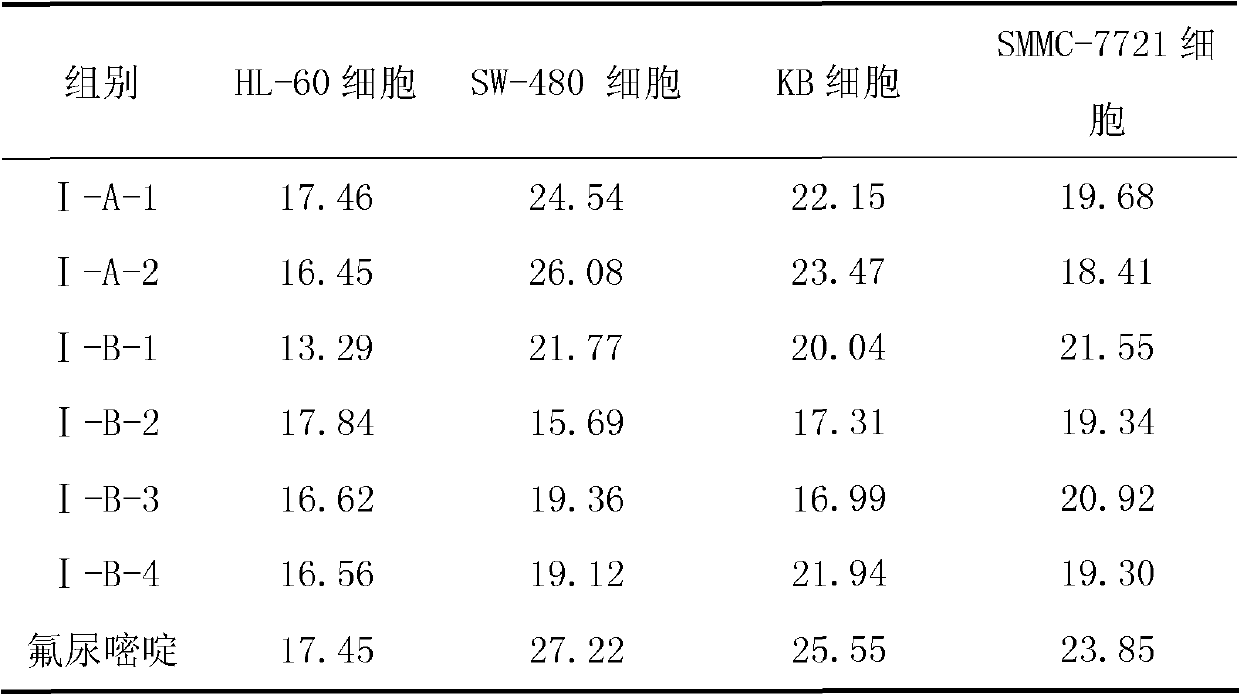
[0068] 5-(pyridin-2-yl-methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (Compound I-A-1)
[0069]
[0070] Add 13.9 g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine into a reaction flask equipped with stirring, condenser and thermometer, dissolve it with 50 mL of acetonitrile, and cool it to -10 °C, 41.5 g of anhydrous potassium carbonate was added. 17.2 g of 2-bromomethylpyridine was added to the reaction system in batches, and after the addition, the temperature was raised to 45° C. to continue the reaction for 3 h (the plate showed that the reaction was complete). Filter, evaporate the filtrate to dryness of acetonitrile, add 50mL of dichloromethane, wash the reaction solution with 3×50mL of water, separate the dichloromethane layer, fully dry it with anhydrous sodium sulfate, filter, and evaporate the dichloromethane under reduced pressure to obtain White solid product 15.7g (HPLC: 97.9%), yield 68.4%. Rf = 0.55 [single site, developer: v (petroleum ether): v (ethyl acetat...
Embodiment 2
[0072] 5-(quinolin-2-yl-methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (Compound I-A-2)
[0073]
[0074] Add 13.9 g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine into a reaction flask equipped with stirring, condenser and thermometer, dissolve it with 80 mL of dichloromethane, and cool it to At 0°C, 30.4 g of triethylamine was added. 17.8 g of 2-(chloromethyl)quinoline was added to the reaction system in batches, and after the addition, the temperature was raised to reflux to continue the reaction for 4 hours (the plate showed that the reaction was complete). Wash the reaction solution with 3×80mL water, separate the dichloromethane layer, fully dry it with anhydrous sodium sulfate, filter, and evaporate the dichloromethane under reduced pressure to obtain 19.9g of white solid product (HPLC: 98.4%), the yield 70.9%. Rf = 0.53 [single site, developer: v (petroleum ether): v (ethyl acetate) = 2: 1]. 1 H NMR (CDCl3, 400MHz) δ: 2.918(s, 4H), 3.684(s, 2H), 4.055(s, 2H), 6.689~...
Embodiment 3
[0076] Intermediate II-B- 1 preparation of
[0077]
[0078] Add 19.2g of 5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one into a reaction flask equipped with stirring, condenser and thermometer, and dissolve it with 65mL of toluene , cooled to 10°C with stirring, and 23.7 g of pyridine was added. 17.2 g of 2-bromomethylpyridine was added to the reaction system in batches, and after the addition, the temperature was raised to 95° C. to continue the reaction for 2 h (the plate showed that the reaction was complete). The reaction solution was washed with 3×70 mL of water, the toluene layer was separated, fully dried with anhydrous sodium sulfate, filtered, and the toluene was evaporated under reduced pressure to obtain a light yellow waxy product (HPLC: 97.4%). Rf=0.51 [single point, developer: v (petroleum ether): v (ethyl acetate) = 1:2]. 1 H NMR (DMSO-d6, 400MHz) δ: 3.072~3.101(t, 2H), 3.578~3.607(t, 2H), 4.248(s, 2H), 4.284~4.301(t, 1H), 4.617(s, 2H) ), 6.577(s, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com