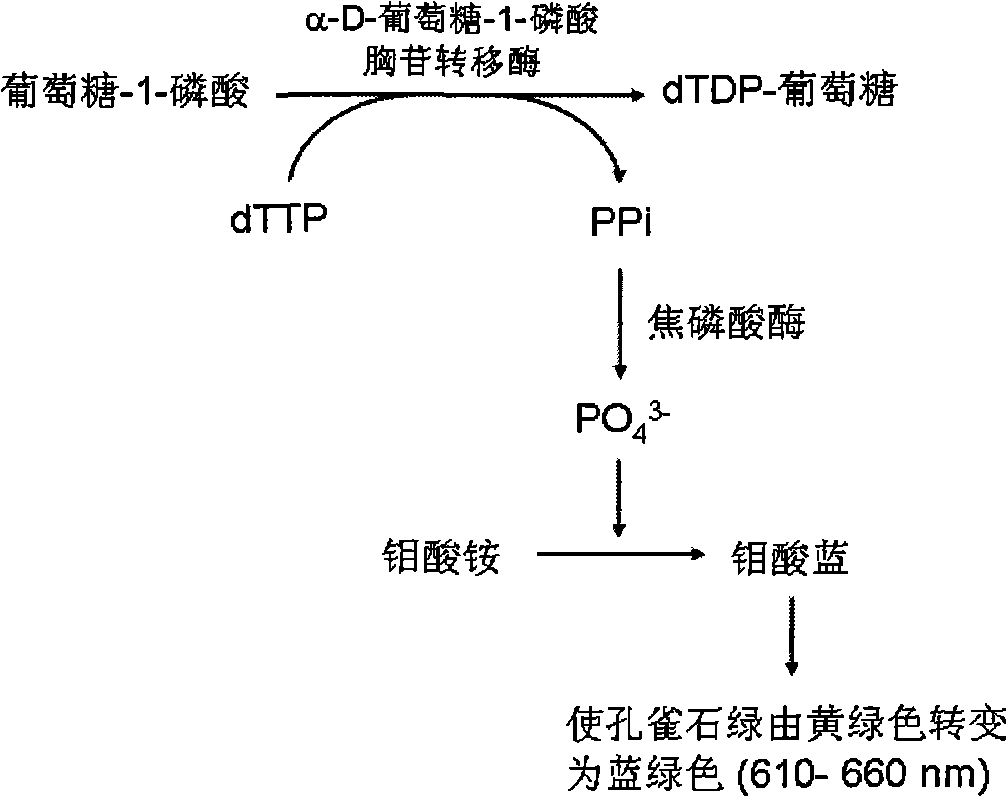
High throughput screening method of mycobacterium tuberculosis alpha-D-glucose-1-thymine phosphate transferase inhibitor
A technology of mycobacterium tuberculosis and thymidine phosphate, applied in the field of screening anti-tuberculosis drugs, can solve problems such as low repeatability, inability to perform high-throughput screening, and large enzymatic reaction system, achieve high drug efficacy, overcome killing Effects on normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] a. Cloning the rmlA gene from the genomic DNA of Mycobacterium tuberculosis H37Rv strain (purchased from American Type Culture Collection, ATCC # is 25618D-5) by molecular cloning technology;
[0021] b. Cloning the rmlA gene into the pET-16b vector (purchased from Novagen, the product number is 69662), and constructing the pET-rmlA expression vector;
[0022] c. Transform the pET-rmlA expression vector into Escherichia coli BL21(DE3) (purchased from Novagen, the product number is 69450), and construct a project expressing Mycobacterium tuberculosis α-D-glucose-1-phosphate thymidine transferase Strain MTRA09 (Escherichia coli MTRA09), deposit number CGMCC 3420;
[0023] d. Inoculate the engineering strain MTRA09 into LB medium containing ampicillin, culture at 37°C for 4 hours, and induce the expression of RmlA protein with a concentration of 0.5mM IPTG;
[0024] e. Harvest the bacteria, break the cells, take the supernatant after centrifugation, and use histidine-Ni ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com