Cell-penetrating peptide (Arg) 9 and lidamycin fusion protein (Arg) 9-LDP
A 9-LDP-AE, fusion protein technology, applied in the direction of peptide/protein components, DNA/RNA fragments, peptides, etc., to achieve the effect of inhibiting tumor growth, good clinical application prospects, and promoting tumor cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
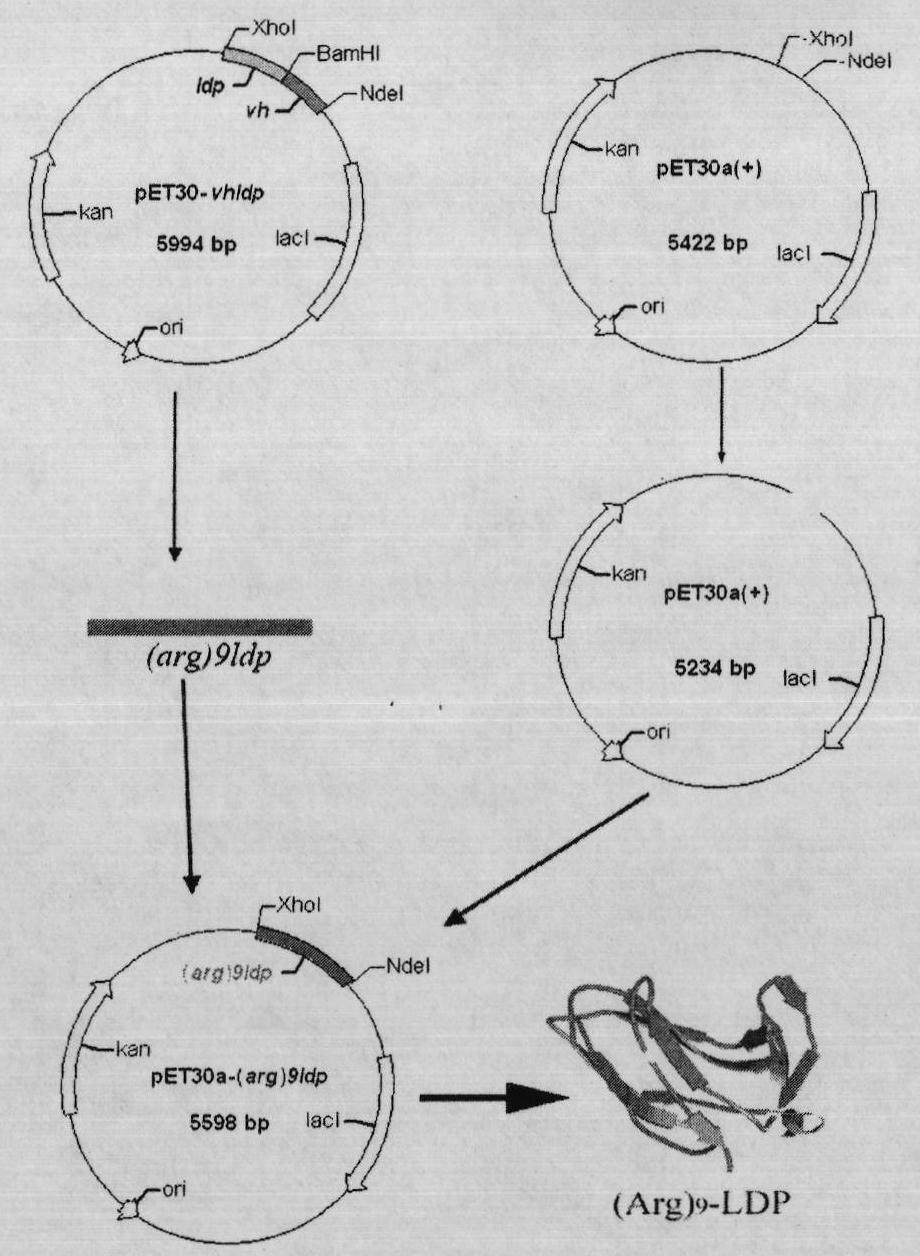
[0146] . Fusion protein (Arg) 9 -LDP recombinant expression plasmid pET30-(arg) 9 build of ldp
[0147] The recombinant plasmid pET30-vhldp (patent: CN200410034052.7) contains the LDP gene. Two restriction sites, NdeI and XhoI, were introduced by PCR (primers were synthesized by Invitrogen).
[0148] Upstream primer: 5′GAATTC CATATG GGCGCGCCCGCCTTCTCCGT 3′(The underlined part is the Nde I restriction site, and the italic part is (arg) 9 Gene)
[0149] Downstream primer: 5′GTTA CTCGAG GCCGAAGGTCAGAGCCACGTG3′ (the underlined part is the Xho I restriction site)
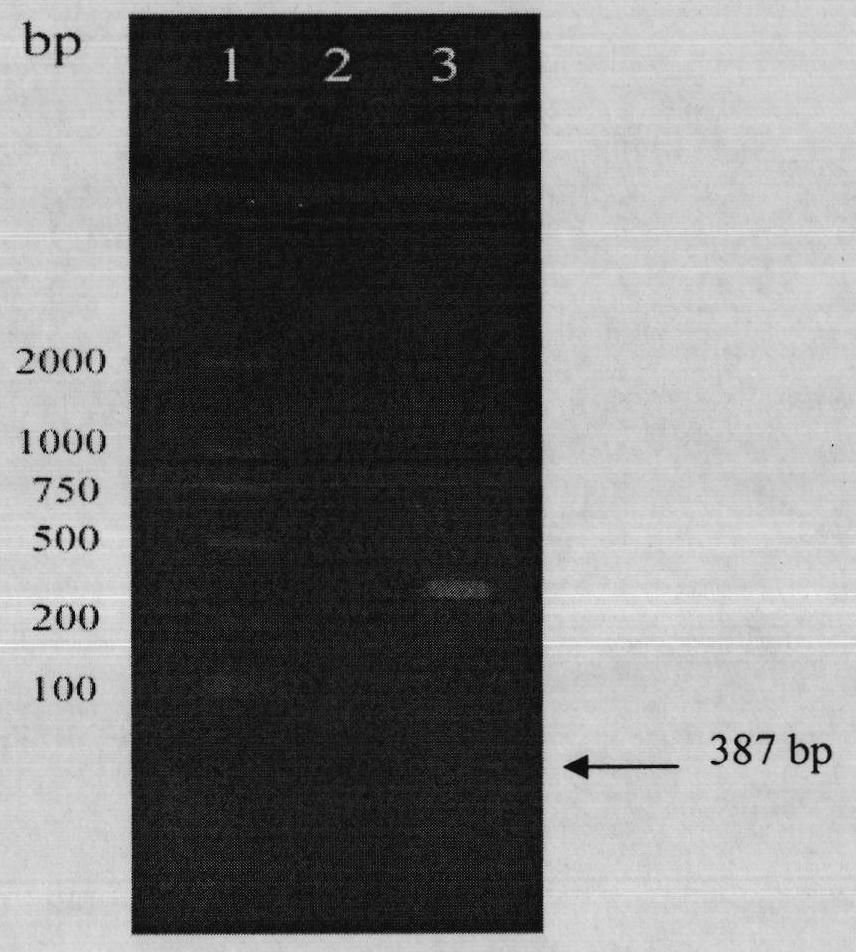
[0150] Recombinant expression plasmid pET30-(arg) 9 The construction process of ldp is as follows figure 1 shown. Carry out PCR amplification with recombinant plasmid pET30-vhldp as template, obtain (arg) 9 ldp gene fragment ( figure 2 ). The PCR reaction system was denaturation at 94°C for 5 minutes, followed by 30 cycles of amplification reaction: denaturation at 94°C for 1 minute, annealing at 58°C fo...
Embodiment 2
[0151] . Fusion protein (Arg) 9 -LDP in Escherichia coli BL21(DE3) star TM inducible expression
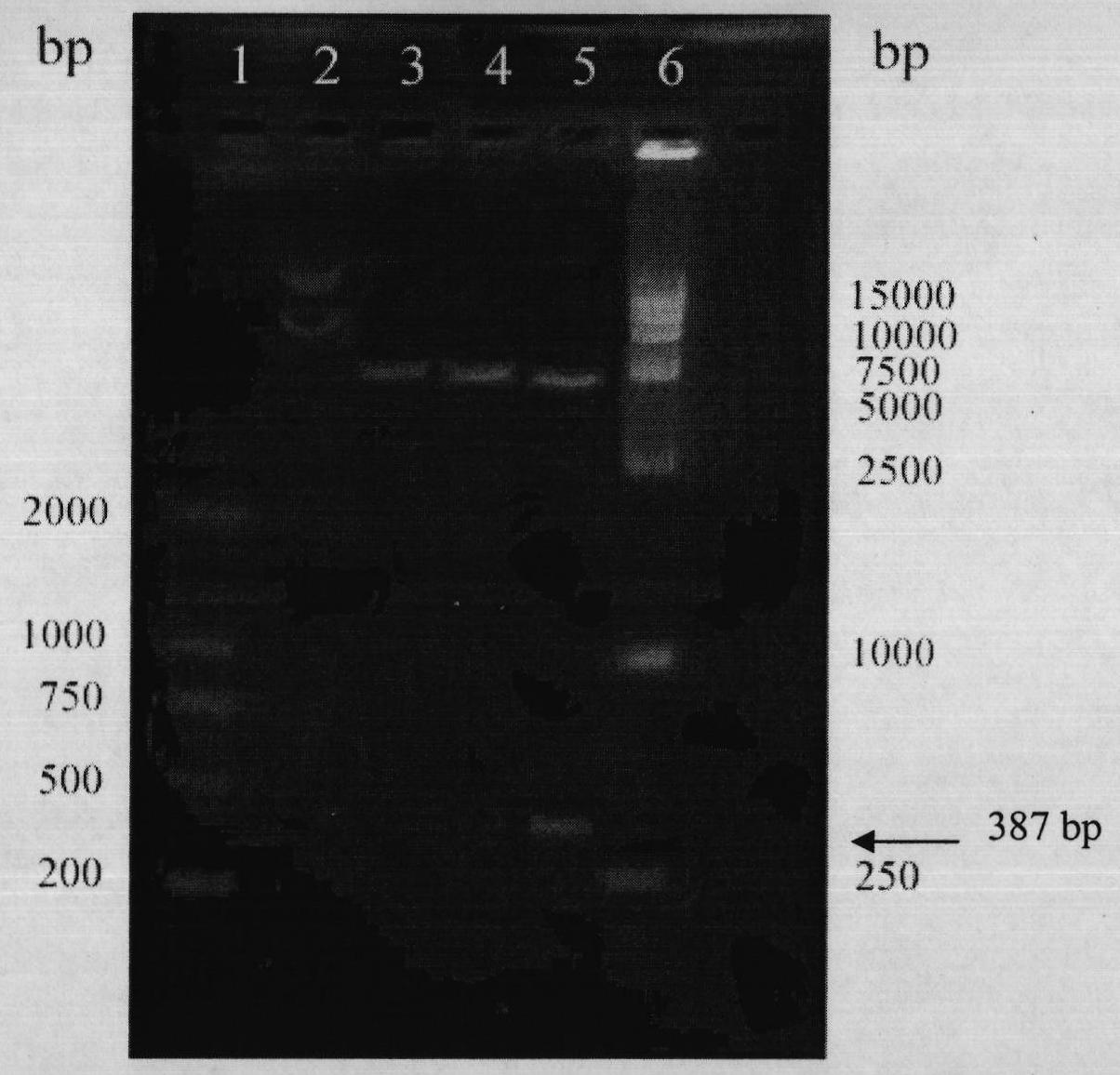
[0152] identified pET30-(arg) 9 Transformation of ldp recombinant plasmid into Escherichia coli BL21(DE3)star TM (Invitrogen company product), randomly pick recombinant transformants and inoculate into 3 ml LB liquid medium containing 50 μg / ml kanamycin, and culture overnight at 37° C. with shaking. The next day, they were inoculated at a ratio of 5%, cultured with shaking at 37°C until the OD600 was 0.8, added IPTG to a final concentration of 0.2mM, and induced for 8 hours. Take an appropriate amount of bacterial liquid, and analyze the expression product localization of the whole bacteria, culture supernatant, periplasmic cavity fraction, soluble cytoplasmic fraction and inclusion body fraction. The results of 15% SDS-PAGE electrophoresis and immunoblotting showed that the fusion protein with a molecular weight of about 14.5kDa was expressed in inclusion bodies in an insolub...
Embodiment 3
[0153] . Fusion protein (Arg) 9 -LDP affinity chromatography purification and separation preparation
[0154] 3.1 Extraction of inclusion body protein
[0155] The culture of recombinant bacteria induced by IPTG was centrifuged at 10000 g for 10 min, the supernatant was removed as much as possible, and the bacteria were collected. Resuspend the cells with 100ml of 20mM Tris-HCl per 1L of culture volume. Sonication disrupts cells. Centrifuge at 10,000 g at 4°C for 15 min to collect inclusion bodies and cell fragment proteins, and discard the supernatant. The pellet was resuspended with 100 ml of urea-free 1×Binding Buffer (20 mM Tris-HCl, 0.5 M NaCl, 5 mM imidazole, pH 8.0). Centrifuge at 10,000 g for 15 min at 4°C, discard the supernatant, and collect the precipitate. Repeat the above operation steps, resuspend the pellet with 50ml 1×Binding Buffer (20mM Tris-HCl, 0.5MNaCl, 5mM imidazole, 8M urea, pH8.0), and completely dissolve the inclusion body protein in ice bath for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com