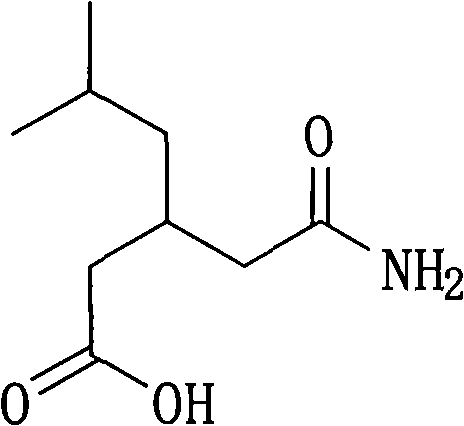
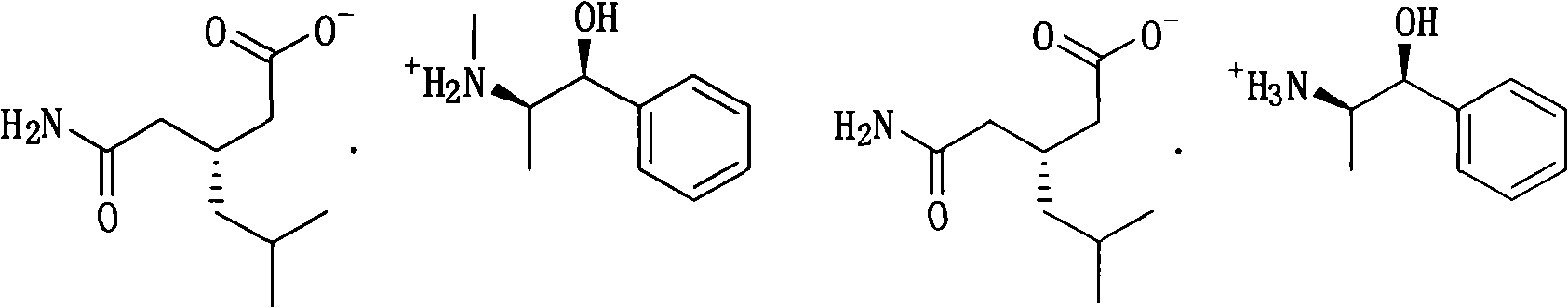Resolution method of 3-(carbamoylmethyl)-5-methylhexanol
A technology of carbamoylmethyl and methylhexanoic acid, applied in the field of splitting of 3-(carbamoylmethyl)-5-methylhexanoic acid, can solve the problem of high price, high environmental pollution, high toxicity, etc. problem, to achieve the effect of simple method, high yield and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]9.35 g (50 mmol) 3-(carbamoylmethyl)-5-methylhexanoic acid, 12 g (50 mmol) (1R,2S)-N,N-dimethyl-1-p-nitro Phenyl-2-amino-1,3-propanediol (dimethylated L-chloramphenicol), 50 ml of water was added to a 100 ml three-necked flask, heated to reflux, all solids were dissolved, stirred for 5 minutes, and slowly cooled to 50°C , kept warm for 3 hours, then lowered to 30°C, filtered, washed with 5 ml of water to obtain (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid and (1R,2S)-N , N-dimethyl-1-p-nitrophenyl-2-amino-1,3-propanediol amine salt 9.8 g, yield 46%.
[0049] diastereoisomeric salts 1 H-NMR (DMSO-d6, 300MHz): δ0.81-0.83 (d, 6H, J=6.4), δ1.08-1.12 (m, 2H), 1.60 (m, 1H), δ1.99-2.22 ( m, 5H), δ2.39(s, 6H), δ2.59-2.61(m, 1H), δ3.36-3.45(m, 2H), δ4.68-4.70(d, 1H, J=7.47) , 5.2 (s, (broad), 3H) δ6.75 (s, 1H), δ7.30 (s, 1H), 7.62-7.64 (d, 2H, J=7.44), 8.16-8.19 (d, 2H, J = 8.54).
[0050] The salt was dissociated by adding 18 ml of water and 25 ml of concentrated hydro...
Embodiment 2
[0052] 9.35 g (50 mmol) 3-(carbamoylmethyl)-5-methylhexanoic acid, 12 g (50 mmol) (1R,2S)-N,N-dimethyl 1-p-nitrobenzene Base-2-amino-1,3-propanediol (dimethylated L-chloramphenicol), 50 ml of water was added to a 100 ml three-necked flask, heated to reflux, all solids were dissolved, stirred for 5 minutes, and slowly cooled to 60 ℃, heat preservation for 1.5 hours, then lowered to 30 ℃, filtered, washed with 5 ml of water to obtain (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid and (1R, 2S)- 10.2 g of N,N-dimethyl-1-p-nitrophenyl-2-amino-1,3-propanediol amine salt, yield 47.8%. After dissociation with hydrochloric acid, (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid is the majority, and the enantioselectivity is 79.0% ee.
[0053] Take 5 grams of (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid and (1R,2S)-N,N-dimethyl-1-p-nitrophenyl- 2-Amino-1,3-propanediol amine salt, recrystallized with water to obtain 4.1 g, yield 82%, after dissociation with hydrochloric acid, o...
Embodiment 3
[0055] 9.35 g (50 mmol) 3-(carbamoylmethyl)-5-methylhexanoic acid, 9.6 g (40 mmol) (1R,2S)-N,N-dimethyl 1-p-nitrobenzene Base-2-amino-1,3-propanediol (dimethylated L-chloramphenicol), 50 ml of water was added to a 100 ml three-necked flask, heated to reflux, all solids were dissolved, stirred for 5 minutes, and slowly cooled to 60 ℃, heat preservation for 3 hours, then lowered to 30 ℃, filtered, washed with 5 ml of water to obtain (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid and (1R, 2S)- 10.5 g of N,N-dimethyl-1-p-nitrophenyl-2-amino-1,3-propanediol amine salt, yield 55%. After dissociation with hydrochloric acid, (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid is the majority, and the enantioselectivity is 65.3%ee.
[0056] Resolution of racemic 3-( Carbamoylmethyl)-5-methylhexanoic acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com