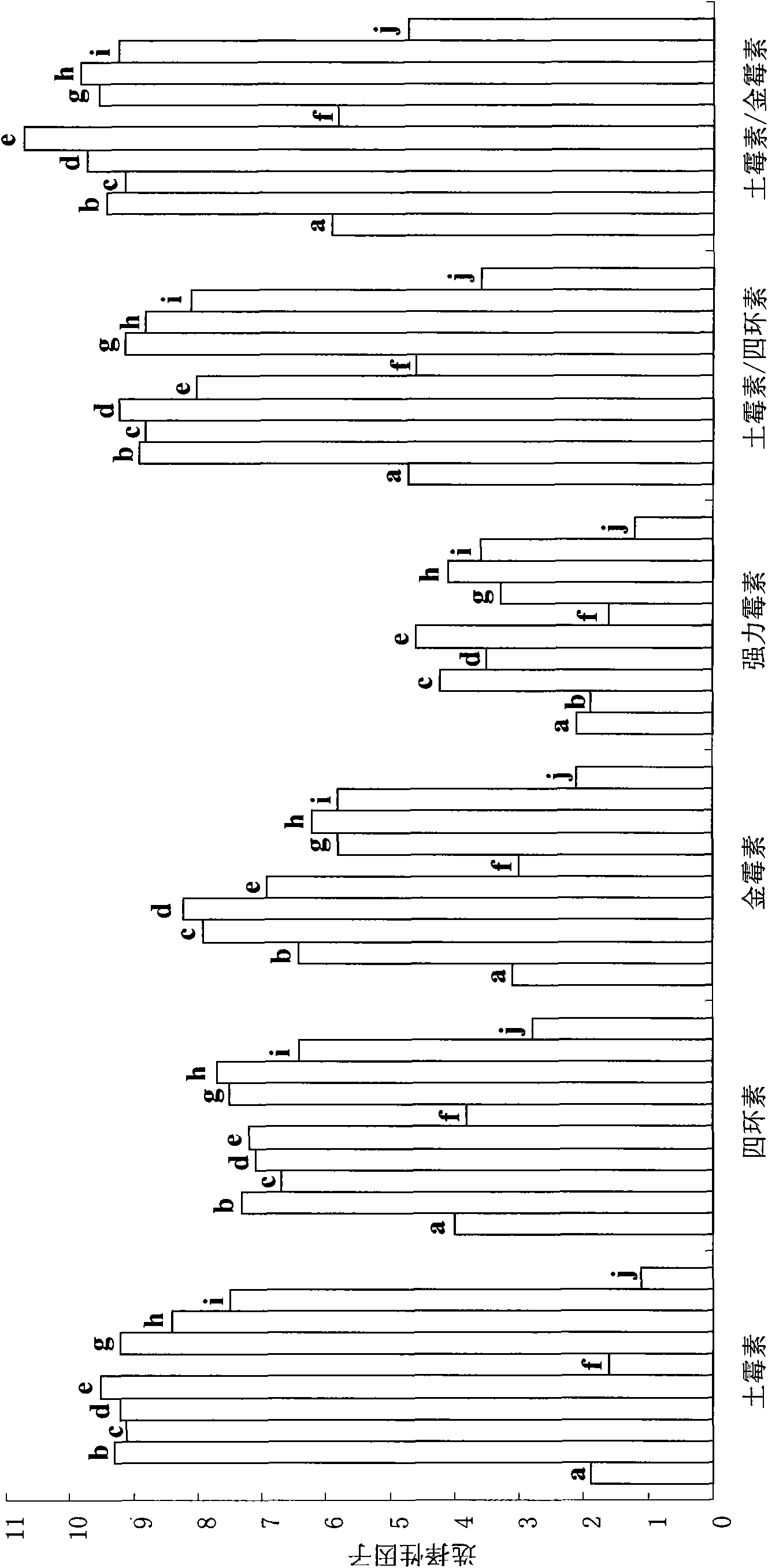
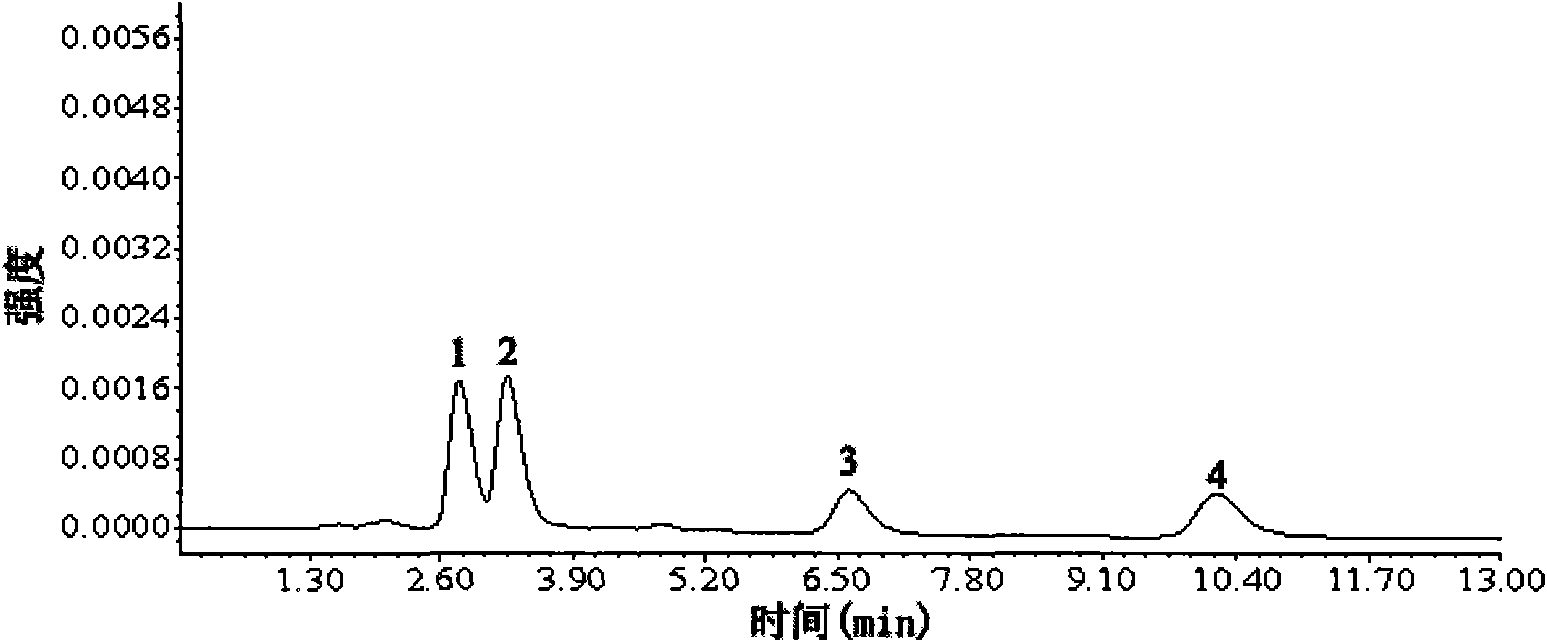
Preparation method of molecular imprinting polymer with specific recognition capability to tetracycline family
A recognition ability and molecular imprinting technology, applied in chemical instruments and methods, other chemical processes, etc., to achieve high sensitivity, good reproducibility, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 1 mmol aureomycin and 1 mmol oxytetracycline as template molecules with 8 mmol functional monomer methacrylic acid in 2 mL containing 40 mmol cross-linking agent N, N'-methylenebisacrylamide and 0.08 mmol initiator azobisiso Prepare a mixed solution in acetonitrile of nitrile; ultrasonically degas the mixed solution for 6 minutes, pass nitrogen gas for 5 minutes to remove oxygen molecules, then seal the mixed solution, and polymerize for 18 hours under stirring at 50°C to form a white powdery imprinted polymer. After the polymerization is completed, use 100 mL of a mixed solution of acetic acid and methanol (wherein the volume percentage concentration of acetic acid is 10%), and use Soxhlet extraction to extract the above-mentioned white powdery imprinted polymer to remove template molecules, and then use methanol Soxhlet extraction method was used to extract and remove acetic acid molecules. After the extraction, the polymer was dried in a vacuum drying oven t...
Embodiment 2
[0028]Dissolve 1mmol aureomycin and 1mmol oxytetracycline as template molecules with 2mmol functional monomer methacrylic acid to 2mL containing 2mmol N,N'-methylenebisacrylamide and 0.02mmol initiator azobisisobutyronitrile Prepare a mixed solution in acetonitrile; the mixed solution was ultrasonically degassed for 5 minutes, nitrogen gas was passed for 10 minutes to remove oxygen molecules, and then the mixed solution was sealed and polymerized for 12 hours under stirring at 45°C to produce a white powdery imprinted polymer. After the polymerization is completed, use 100 mL of a mixed solution of acetic acid and methanol (wherein the volume percentage concentration of acetic acid is 10%), and use Soxhlet extraction to extract the above-mentioned white powdery imprinted polymer to remove template molecules, and then use methanol Extraction removes acetic acid molecules. After the extraction, the polymer was dried in a vacuum drying oven to a constant weight, and finally a mol...
Embodiment 3
[0030] Dissolve 1mmol aureomycin and 1mmol oxytetracycline as a template molecule with 20mmol functional monomer methacrylic acid in 20mL containing 200mmol N,N′-methylenebisacrylamide and 2mmol initiator azobisisobutyronitrile Prepare a mixed solution in acetonitrile; ultrasonically degas the mixed solution for 10 minutes, pass nitrogen gas for 10 minutes to remove oxygen molecules, then seal the mixed solution, and polymerize for 24 hours under stirring at 60°C to generate a white powdery imprinted polymer. After the polymerization is completed, use 100 mL of a mixed solution of acetic acid and methanol (wherein the volume percentage concentration of acetic acid is 20%), and use Soxhlet extraction to extract the above-mentioned white powdery imprinted polymer to remove template molecules, and then use methanol Extraction removes acetic acid molecules. After the extraction, the polymer was dried in a vacuum drying oven to a constant weight, and finally a molecularly imprinted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com