Lactide/epsilon-caprolactone copolymer for medical implant, method for producing lactide/epsilon-caprolactone copolymer for medical implant, medical implant and artificial dura mater
A lactide and caprolactone technology, applied in the field of medical implants and artificial dura mater, can solve the problems of loss of flexibility and hesitation in use, and achieve high flexibility, high mechanical strength, high safety and high suture strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
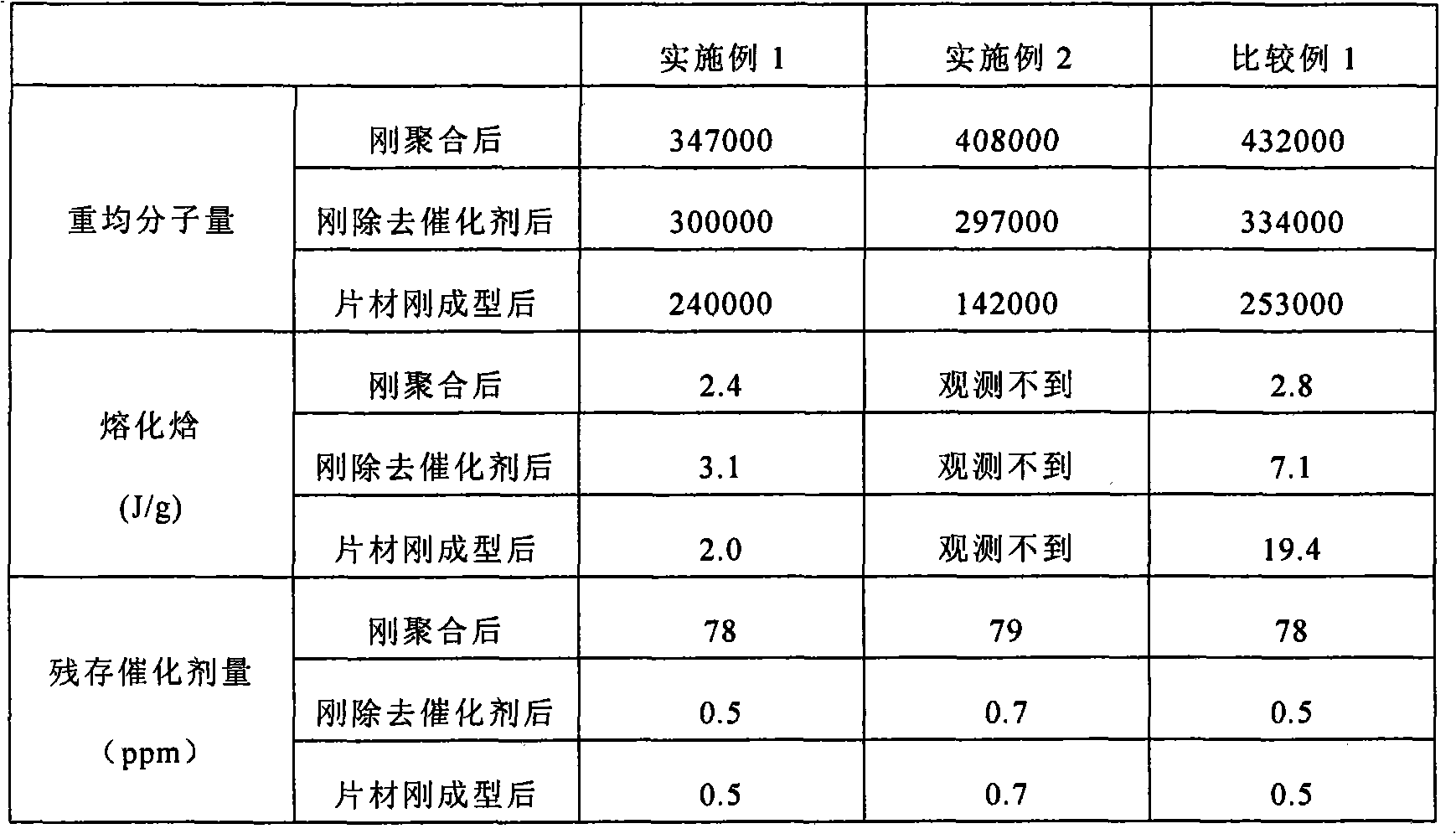
Embodiment 1
[0061] 1440 g of L-lactide, 1140 g of ε-caprolactone, and 300 ppm of tin octoate (78 ppm in terms of tin) were placed in a detachable flask, and polymerized at 135° C. for 7 days under a nitrogen atmosphere after decompression.
[0062] The obtained copolymer was treated with a catalyst remover (acetic acid / isopropanol=30 / 70 v / v), and vacuum-dried at 40°C to obtain a lactide / ε-caprolactone copolymer.
[0063] The obtained lactide / ε-caprolactone copolymer was melt-molded with an extruder into a sheet with a thickness of 100 μm, and then heat-treated at 70° C. for 12 hours in a vacuum to obtain a sheet.
Embodiment 2
[0065] Put 1,440 g of L-lactide, 1,140 g of ε-caprolactone, and 300 ppm of tin 2-ethylhexanoate (78 ppm in terms of tin) into a detachable flask, and polymerize at 140°C under a nitrogen atmosphere after decompression for 7 sky.
[0066] The obtained copolymer was treated with an acetic acid-isopropanol solution (acetic acid / isopropanol=20 / 80 v / v), and vacuum-dried at 40°C to obtain a lactide / ε-caprolactone copolymer.
[0067] A sheet was obtained in the same manner as in Example 1 except that the obtained lactide / ε-caprolactone copolymer was used.
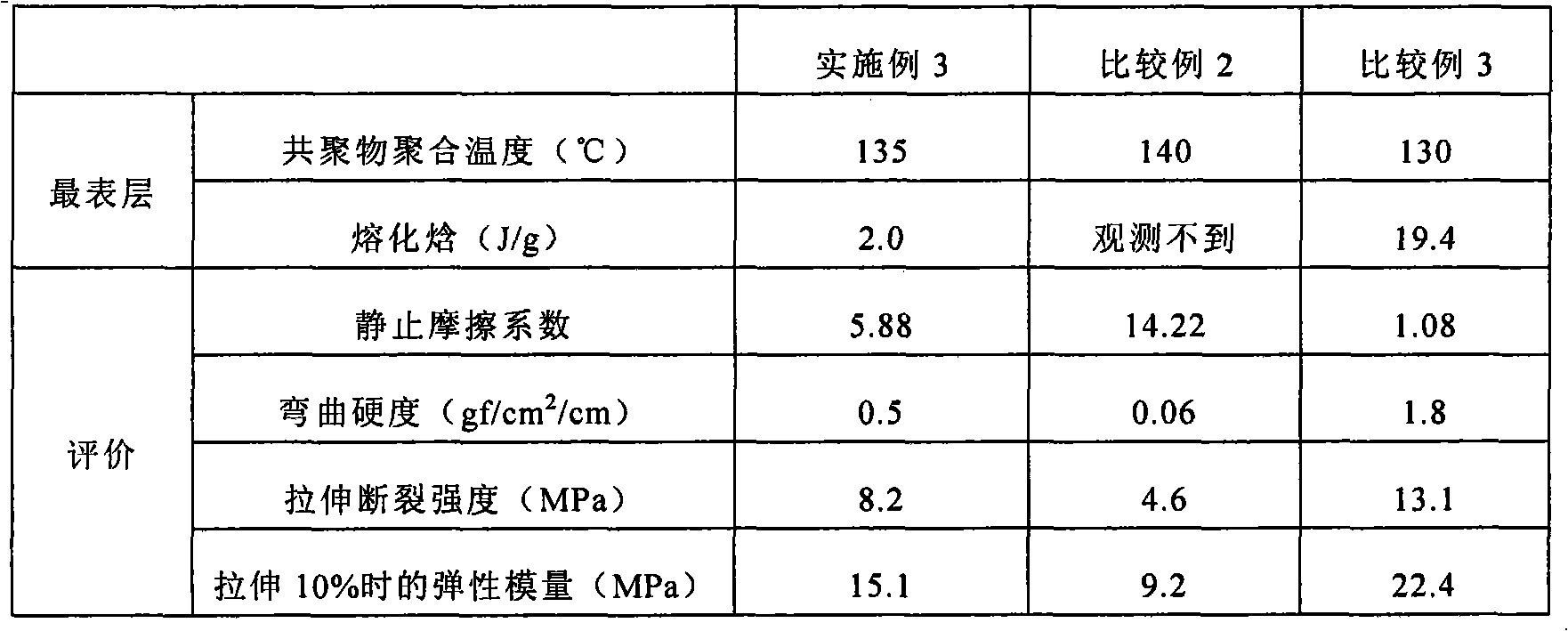
Embodiment 3
[0085] (1) Synthesis of lactide / ε-caprolactone copolymer
[0086] 1440 g of L-lactide, 1140 g of ε-caprolactone, and 300 ppm of tin 2-ethylhexanoate were placed in a detachable flask, and polymerized at 135° C. for 7 days under a nitrogen atmosphere after decompression.
[0087] The obtained lactide / ε-caprolactone copolymer was treated with an acetic acid-isopropanol solution (acetic acid / isopropanol=30 / 70 v / v), and vacuum-dried at 40°C.
[0088] The weight average molecular weight of the obtained copolymer was 320,000 (measured by GPC), and the metal content was less than 0.5 ppm (quantified by ICP emission spectroscopic analysis).
[0089] (2) Preparation of the outermost sheet
[0090] The obtained copolymer was melt-molded with an extruder to obtain a sheet having a thickness of 100 μm.
[0091] An 8 mg sample was cut out from the obtained sheet, and subjected to DSC measurement (DSC temperature increase rate: 10° C. / min), and the fusion enthalpy derived from the crystal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com