Polyfluoroalkyliodide and method of producing the same
A polyfluoroalkyl group and a manufacturing method technology, applied in the preparation of hydroxyl compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low bioaccumulation of compounds, difficulty in manufacturing or use, and high bioaccumulation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] Next, the present invention will be described with reference to examples.
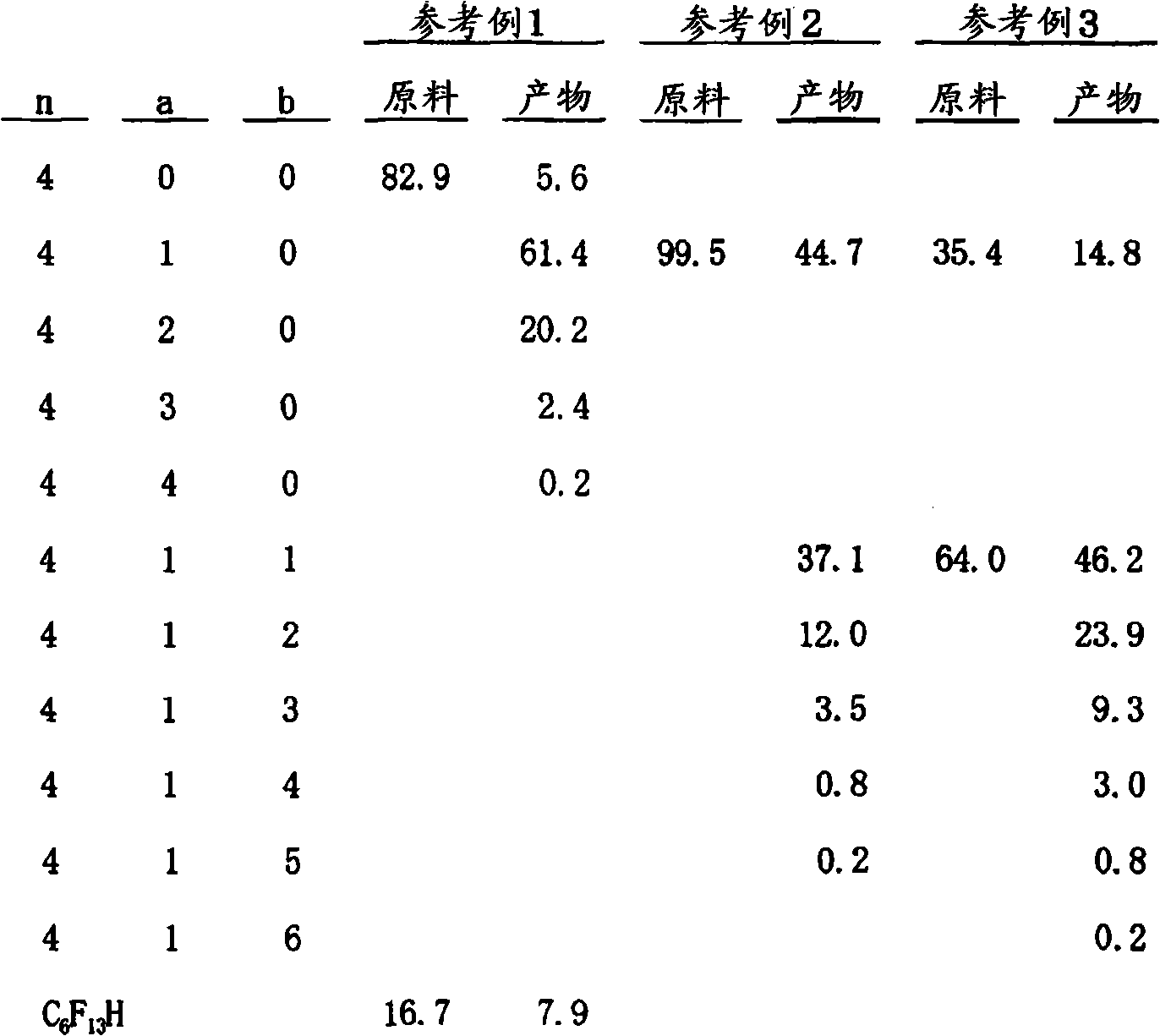
reference example 1
[0042] Perfluorobutyl iodide C 4 f 9 I (purity 82.9%) 500g is packed in the autoclave of capacity 1200ml, when raising its internal temperature to reach about 50 ℃, add and dissolve in C 4 f 9 Two (4-tert-butylcyclohexyl) peroxycarbonate initiator of 160g (Chemical medicine Akuzo product バリッツッツックス 16) 0.75g, when the internal temperature reaches 55°C, keep the pressure of 0.5~0.7MPa while dividing After adding vinylidene fluoride in batches up to 214 g, aging was performed at a temperature of 55-65° C. for 1 hour to terminate the reaction. After the reaction was finished, it was cooled, and 583 g of the product was recovered.
[0043] In addition, the resulting product was separated by distillation under the conditions of a tower top temperature of 58°C and a pressure of 7.4kPa (56mmHg) to obtain CF 3 (CF 2 ) 3 (CH 2 CF 2 ) I (purity 99.5%) 203g. This was used as the reaction raw material of Reference Examples 2 and 3. It should be noted that for the reaction product...
reference example 2
[0045] Will CF 3 (CF 2 ) 3 (CH 2 CF 2 )I (purity 99.5%) 600g is packed in the autoclave of capacity 1200ml, when raising its internal temperature to reach about 50 ℃, add and dissolve in CF 3 (CF 2 ) 3 (CH 2 CF 2 ) 1.35g of the peroxide system initiator (bakadox 16) of 1 300g, after internal temperature reaches 55 ℃, while keeping the pressure of 0.2~0.3MPa, add tetrafluoroethylene in batches, after adding 150g in batches, Aging at 55-74° C. for 1 hour to end the reaction. After the reaction was finished, it was cooled, and 1010 g of the product was recovered.
[0046] In addition, the resulting product was separated by distillation under the conditions of a tower top temperature of 71°C and a pressure of 2.6kPa (20mmHg), to obtain CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 ) I (purity 99.8%) 347g. This was used as the reaction raw material of Reference Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com