Itraconazole gel for dogs and preparation method thereof
An itraconazole gel and the technology of itraconazole are applied in the field of itraconazole gel for dogs and its preparation, which can solve the problems of long treatment process, large dosage, skin damage, etc., and achieve good therapeutic effect, Small toxic and side effects, non-toxic effect on the skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
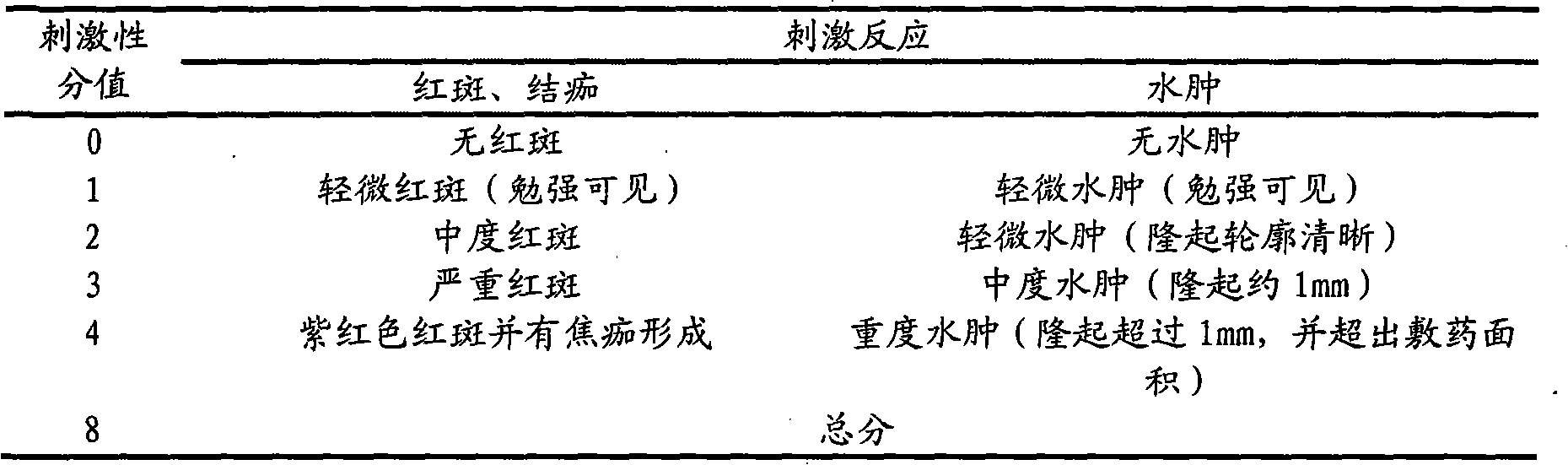
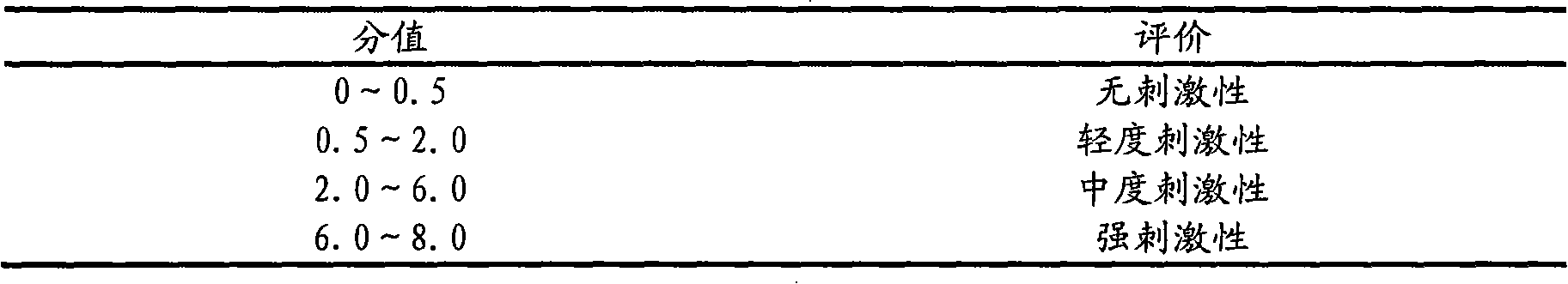
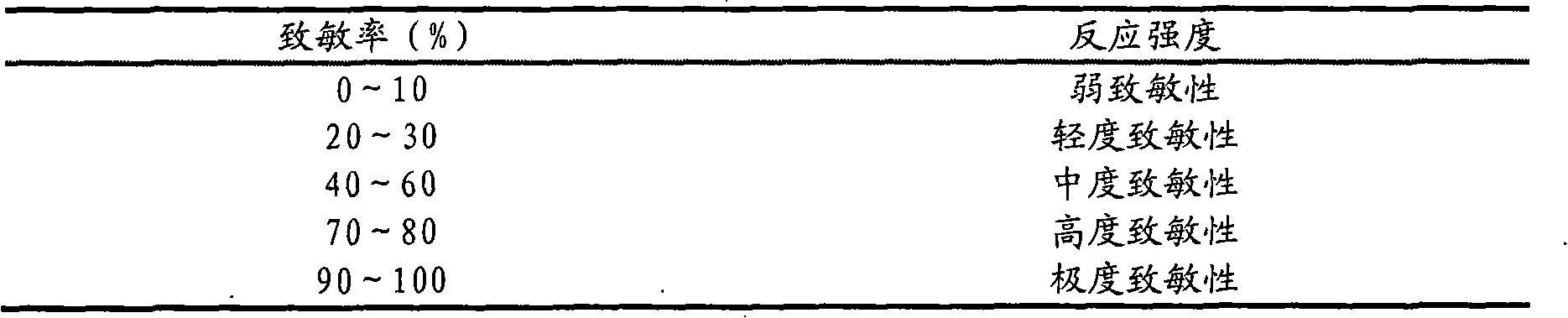
[0016] Accurately weigh 1.5g of itraconazole, add absolute ethanol to the Erlenmeyer flask, and add concentrated hydrochloric acid dropwise under magnetic stirring to completely dissolve to a transparent liquid; then weigh 52.5g of hydroxypropyl-β-cyclodextrin, Add distilled water to dissolve in a water bath at 60°C to obtain a hydroxypropyl-β-cyclodextrin solution; slowly add the hydroxypropyl-β-cyclodextrin solution to the itraconazole acidic solution under magnetic stirring, dropwise The time is 30 minutes, 24 hours at 40°C to obtain the clathrate solution; another 1.5 g of Carbomer 940 is weighed and placed in a small beaker, spread evenly, spray an appropriate amount of distilled water with a sprayer, and swell for 24 hours to obtain translucent jelly-like object; add ethylene glycol-400 10g, salicylic acid 6g and ethylparaben 0.1g to the swollen carbomer 940 and stir evenly, then slowly add the clathrate solution to the gel , stir until there is no clot, add an appropria...
Embodiment 2
[0018] Accurately weigh 3.0g of itraconazole, add absolute ethanol to the Erlenmeyer flask, and add concentrated hydrochloric acid dropwise under magnetic stirring to completely dissolve to a transparent liquid; then weigh 105.0g of hydroxypropyl-β-cyclodextrin, Add distilled water to dissolve in a water bath at 60°C to obtain a hydroxypropyl-β-cyclodextrin solution; slowly add the hydroxypropyl-β-cyclodextrin solution to the itraconazole acidic solution under magnetic stirring, dropwise The time is 60 minutes, 24 hours at 40°C to obtain the clathrate solution; another 3.0 g of Carbomer 940 is weighed and placed in a small beaker, spread evenly, spray an appropriate amount of distilled water with a sprayer, and swell for 24 hours to obtain translucent jelly-like object; add 20g of ethylene glycol-400, 12.0g of salicylic acid and 0.2g of ethylparaben to the swollen carbomer 940 and stir evenly, then slowly add the clathrate solution dropwise to the condensed In the glue, stir e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com