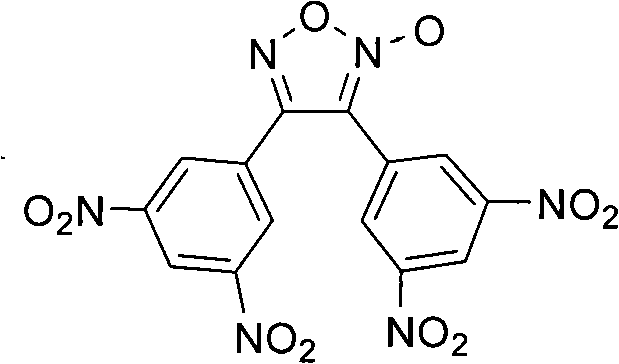
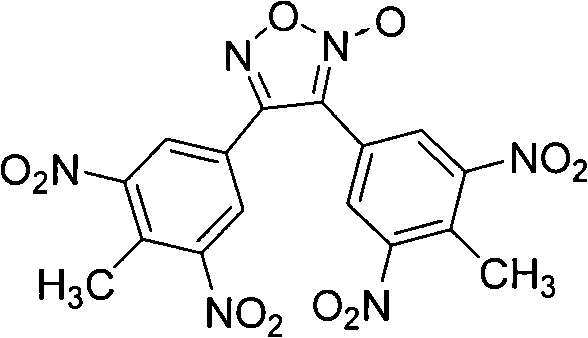
3,4-di(3',5'-dinitryl-4'-methyl phenyl) furoxan compound
A technology of methyl phenyl and furoxan, which is applied in 3 fields, can solve the problems of small thermal decomposition heat release and low thermal stability, and achieve the effect of increasing the thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
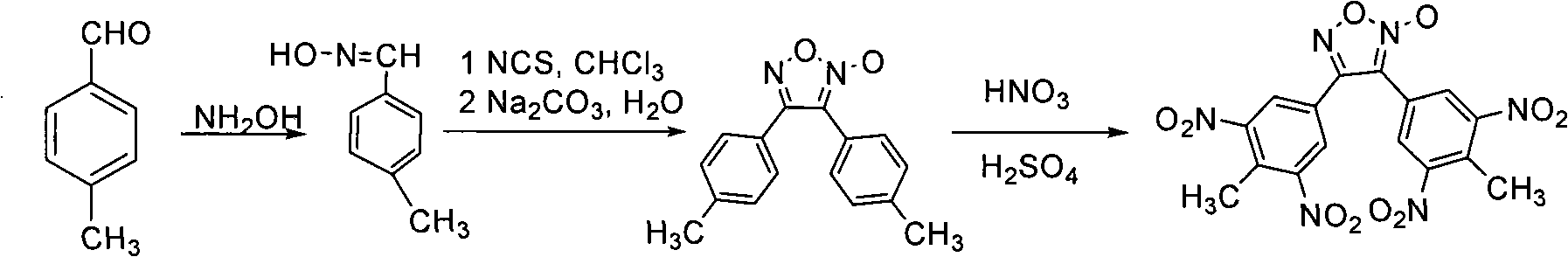
[0017] Example 13, 4-bis (4'-methyl-3', 5'-dinitrophenyl) synthesis of furoxan
[0018] Add 12g (0.1mol) of p-tolualdehyde and 60mL of ethanol in sequence in the reaction flask with mechanical stirring, thermometer and reflux device, then add an aqueous solution containing 10.4g of hydroxylamine hydrochloride, and adjust the reaction solution to neutral with sodium carbonate solution , react at a temperature of 20°C to 30°C for 1 to 2h, after the reaction is completed, filter, wash, and dry to obtain 10.8g of p-tolualdehyde oxime. Yield 80%.
[0019] In a 250 mL three-necked flask, add 2.1 g (0.015 mol) of p-toluene aldoxime and 20 mL of chloroform, dissolve the toluyl aldoxime in chloroform under magnetic stirring, and add 2.0 g (0.016 mol) of chlorinated For succinimide, react at a temperature of 25°C to 30°C for 2 hours, and track it with TLC. After the reaction is complete, use an ice-water bath to lower the temperature of the reaction system to 0°C, and use dilute sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com