Polyester polycondensation catalyst and preparation method and application thereof
A polycondensation catalyst and catalyst technology, applied in the field of polyester polycondensation catalyst and its preparation and application, can solve problems affecting product performance, achieve the effects of suppressing side reactions, high brightness, and improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, preparation polyester polycondensation catalyst
[0025] Take 5mL concentrated hydrochloric acid, 10mL ethanol, mix with 19.6g tetraethyl orthosilicate (TEOS) at room temperature, stir for 1 hour to obtain the product A SiO 2 Sol.
[0026] Take 2.5g tetrabutyl titanate at room temperature and mix with 10mL concentrated hydrochloric acid, stir at room temperature for 1 hour to obtain the product BTiO 2 Sol.
[0027] The product A SiO 2 Sol and product B TiO 2 The sols were mixed and stirred at 50°C for 12 hours to obtain the product CTiO 2 / SiO2 Sol.
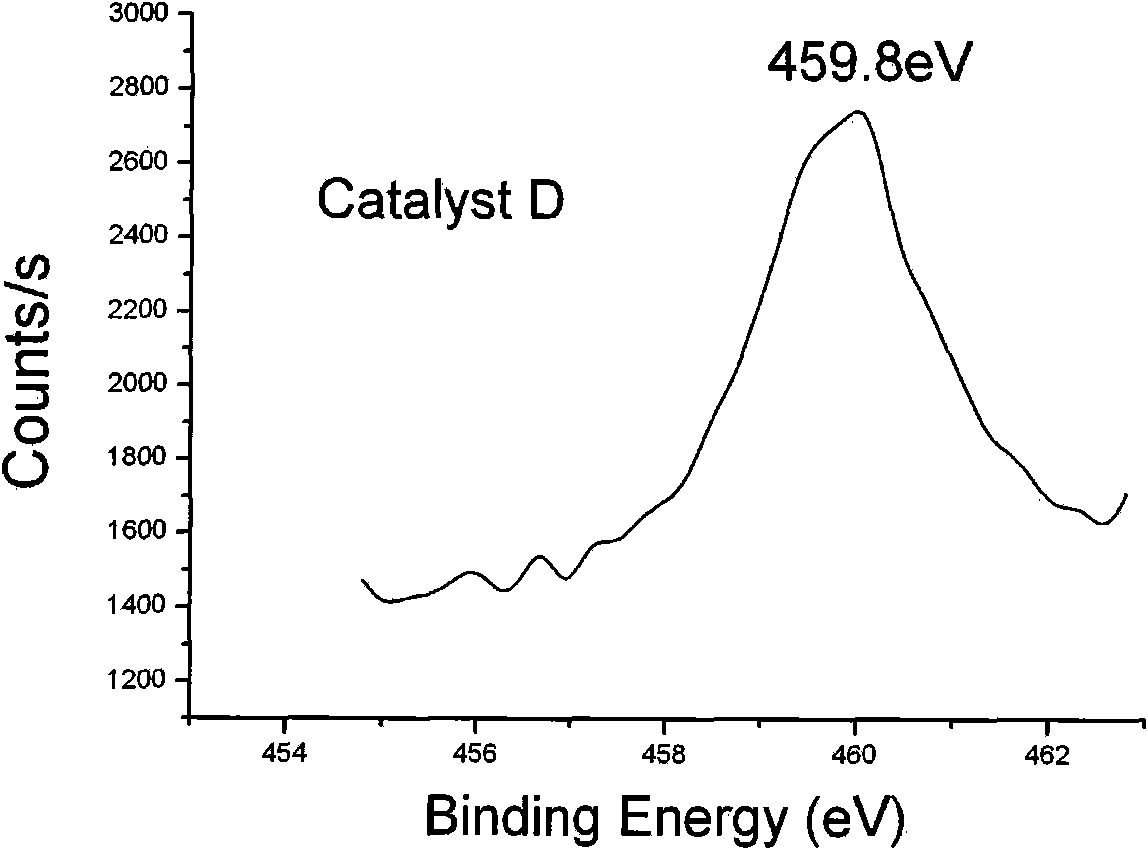
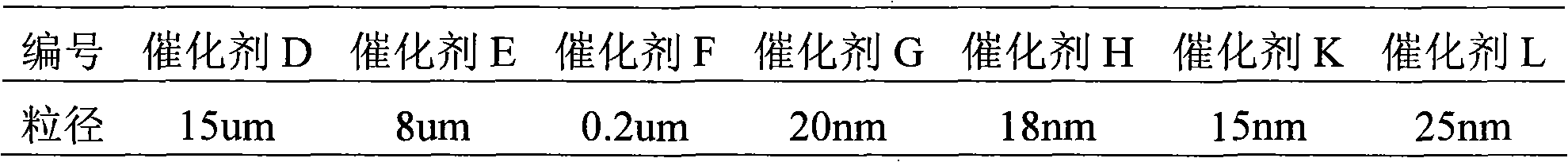
[0028] At 80°C, the product C TiO 2 / SiO 2 The sol was vacuum-dried to obtain catalyst D.
[0029] The product C TiO 2 / SiO 2 The sol was freeze-dried to obtain catalyst E.
[0030] The product C TiO 2 / SiO 2 The sol was loaded into a semipermeable membrane and placed in deionized water to remove Cl - , to obtain catalyst F.
[0031] The product C TiO 2 / SiO 2 Catalyst G was obtained by react...
Embodiment 2
[0034] Embodiment 2, preparation polyester polycondensation catalyst
[0035] Take 5mL concentrated hydrochloric acid, 10mL ethanol, mix with 19.6g tetraethyl orthosilicate (TEOS) at room temperature, stir for 1 hour to obtain the product A SiO 2 Sol.
[0036] Take 2.5g TiCl 4 Add dropwise into 20ml EG at 0°C, and pass through dry NH 3 The gas was first prepared according to the Nelles method provided in the following literature, but the product was not hydrolyzed, and then supported on SiO 2 Above the Sol: Li Dacheng, Zhou Dali, Chen Chaozhen, etc. Synthesis of Titanium Ethoxide and Preparation of TiO by Hydrolysis 2 Research on Micropowder [J]. Functional Materials, 1995, 26(3): 278-282.
[0037] In the above preparation steps, the resulting product was quickly added to A to obtain TiO 2 / SiO 2 Sol J.
[0038] Freeze-dry J under vacuum to obtain catalyst K.
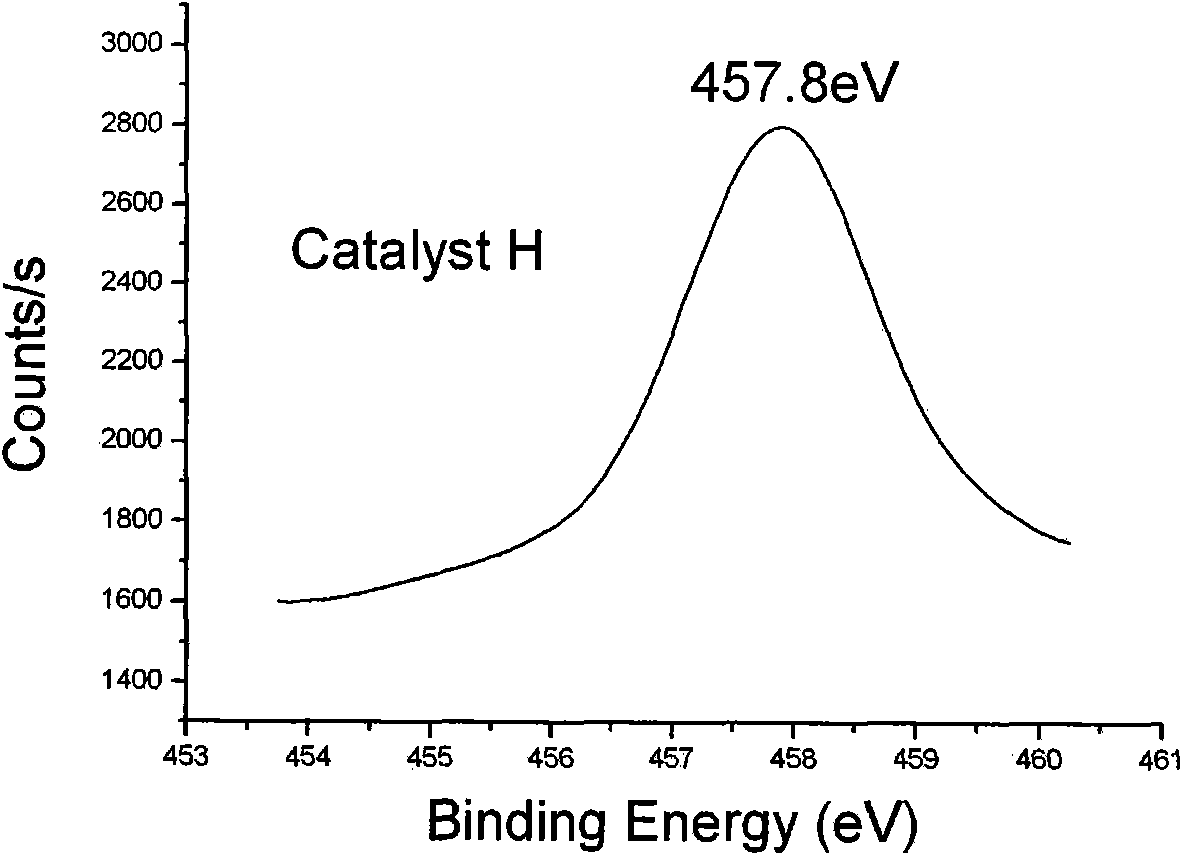
[0039] Treat J with a low-temperature hydrothermal method, the hydrothermal temperature is 50-200° C., and t...
Embodiment 3
[0046] Embodiment 3, the application of polyester polycondensation catalyst as catalyst in the preparation of polyester materials
[0047] Utilize the polyester polycondensation catalyst that the embodiment of the present invention 1 provides to prepare the steps of PET polyester material as follows:
[0048] Take 86.4g of terephthalic acid and 32.3g of ethylene glycol, and carry out esterification at a pressure of 0.3MPa and a temperature of 220-250°C until the theoretical value of water is distilled off. Add 1 mg of one of the catalysts (catalyst D-I) prepared in Example 1 to obtain PET materials N-S respectively. Polycondensation time and product properties are shown in Table 2.
[0049] It can be seen from the data in Table 2 that the electronic binding energy of titanium and the particle size of the catalyst greatly affect the activity of the catalyst: for catalysts D, E, and F, the particle size decreases in turn, the activity increases, and the product performance is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com