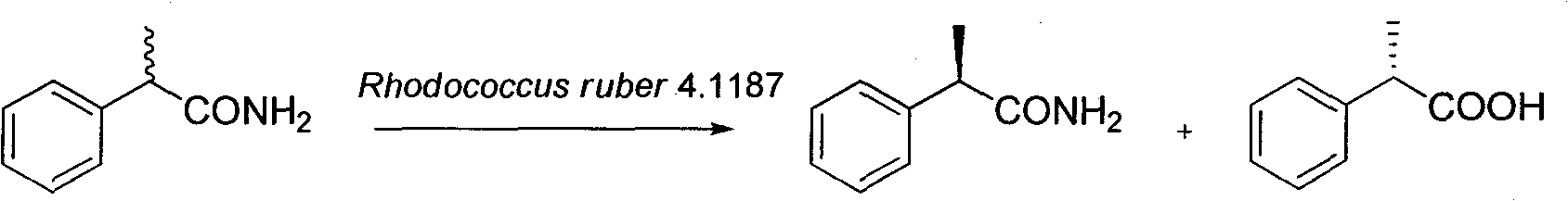
Method for catalytically preparing optically pure (S)-(+)-2-phenylpropionic acid with Rhodococcus ruber 4.1187
A technology for the preparation of phenylpropionic acid and catalysis, which is applied in the direction of biochemical equipment and methods, bacteria, and methods based on microorganisms, can solve the problem of less 2-phenylpropionic acid, and achieve mild reaction conditions, environmental friendliness, and special strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Case 1
[0020] Add 10g Rhodococcus ruber 4.1187 resting cells to 100mL buffer (pH 7.5Tris-HCl, 100mM), the substrate concentration of racemic 2-phenylpropanamide is 20mM (0.30g), and the cosolvent DMSO (5% ), at 30°C, 180rpm in a shaker, the reaction was completed for 3h. The cells were removed by centrifugation, and the supernatant was adjusted to pH 12 with sodium hydroxide solution, extracted three times with the same volume of ethyl acetate, washed with saturated saline, dried over anhydrous sodium sulfate, and concentrated to obtain (R)-(-)-2- Phenylpropanamide; the pH value of the aqueous phase was adjusted to 2 with 2M hydrochloric acid, and the remaining operations were the same as above, and concentrated to obtain (S)-(+)-2-phenylpropionic acid.
[0021] Finally, the yield of product (S)-(+)-2-phenylpropionic acid is 48%, e.e. is 98.5%; the yield of residual substrate (R)-(-)-2-phenylpropanamide is 49% %, e.e. 98%.
[0022] Case 2
[0023] Add 10g of Rhodo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com