Method for directly synthesizing aniline from benzene and ammonia by one step
A benzene one-step, direct technology, applied in chemical instruments and methods, preparation of amino-substituted hydrogen atoms, molecular sieve catalysts, etc., can solve the problems of complex catalyst preparation process, unsatisfactory yield and other problems, and achieve short catalytic reaction time, low price, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 weighs 0.1143g NiSO 4 , 0.1007g CuSO 4 ·5H 2 O, 0.0795g CeN 5 o 9 ·6H 2 O, 0.1004g VOSO 4 ·nH 2 O, 0.5127g TiCl 3 ·nH 2 O (35%) was dissolved in 2 mL of deionized water, heated and stirred until completely dissolved, and set aside. Weigh 5 parts of 1g TS-1 powder and place them in 5 branch test tubes respectively, and vacuumize at 25°C for 0.5 hours, the vacuum degree is 0.9×10 2 KPa, while maintaining the vacuum, pour 5 parts of metal salt solutions that have been prepared into the carriers in 5 branched test tubes while hot, and leave to age for 24 hours; °C until completely dry, then move the dried catalyst into a crucible and bake at 500 °C for 4 hours. Catalysts are marked as Ni / TS-1, Cu / TS-1, Ce / TS-1, V / TS-1, Ti / TS-1, and the weight percentage of the metal loading of the above five catalysts is 2.5%, and they are stored dry for later use .
Embodiment 2
[0019] Embodiment 2 weighed 0.2286g, 0.3429g, 0.4572g NiSO 4 According to the method of Example 1, Ni / TS-1 catalysts were prepared, wherein the nickel loading mass percentages were 5.0%, 7.5%, and 10.0%, respectively.
Embodiment 3
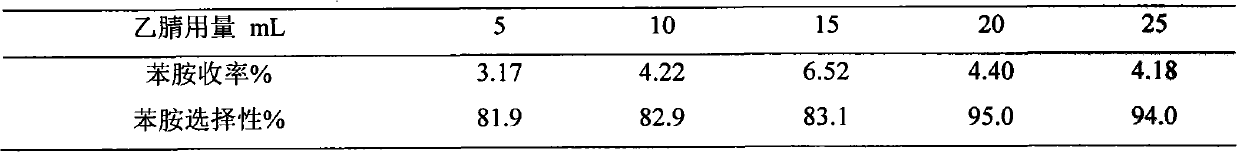
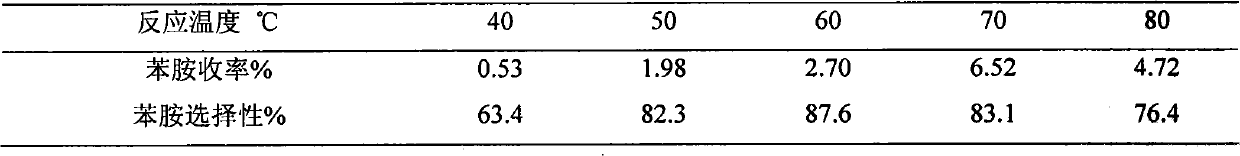
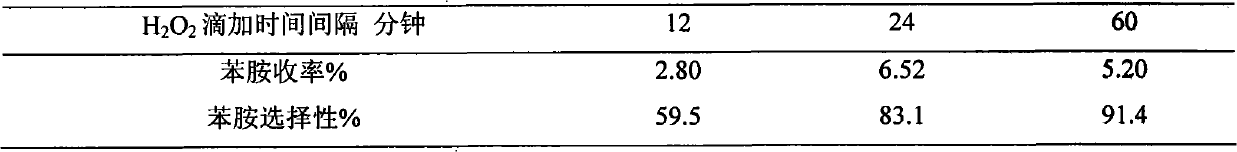
[0020] Example 3 Weigh TS-1, Ni / TS-1, V / TS-1, Cu / TS-1, Ce / TS-1, Ti / TS-1 respectively (wherein the metal loading mass percent is 2.5%) 0.3g into six 50mL two-necked bottles, add 5mL of benzene and 10mL of 25% ammonia water, stir and heat to 70°C, add 2mL of 30% H 2 o 2 , after which 2 mL of 30% H was added every 24 min 2 o 2 , adding a total of 10 mL of 30% H 2 o 2 , the reaction time was 2 hours, and the effects of different metal-loaded catalysts on the aniline yield and aniline selectivity were obtained. The results are shown in Table 1.
[0021] Table 1 Effect of different metal-loaded catalysts on the yield and selectivity of aniline
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com