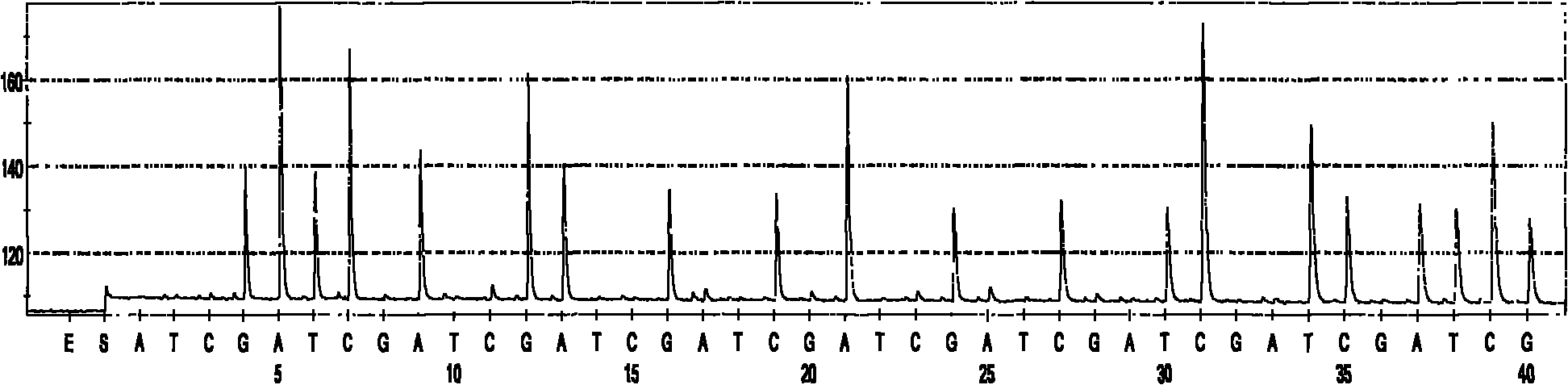
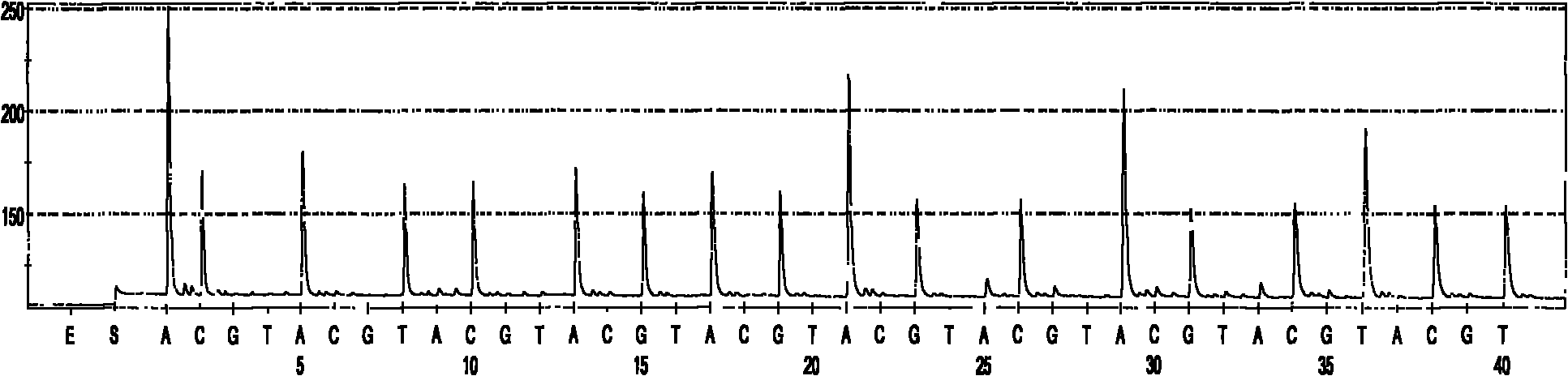
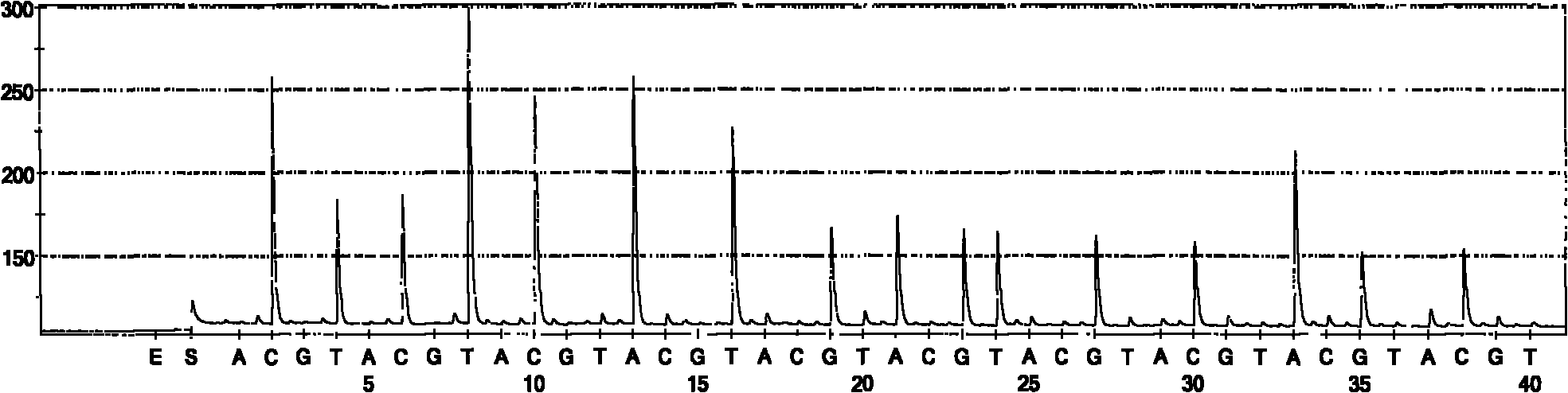
Quick identification kit for bacteria and detection method thereof
A detection method and technology of a kit are applied in the field of clinical microorganism identification, which can solve the problems of high cost and affect the effect of diagnosis and treatment, and achieve the effects of convenient operation, low cost and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation method of kit.
[0055] (1) DNA extraction reagents:
[0056] 5% (w / v, i.e. g / mL) Chelex 100 buffer: containing 0.03% (w / v, i.e. g / mL) SDS (purchased from Hangzhou Fluoride Biotechnology Co., Ltd.), 1% (v / v) Tween-20 (purchased from U.S. Sigma Company), 1% (v / v) NP-40 (purchased from U.S. Sigma Company),
[0057] 20 mg / mL proteinase K: (purchased from Amresco).
[0058] (2) Reaction solution:
[0059] PCRBuffer: 0.1% (v / v) NP-40, 0.02% (v / v) gelatin (purchased from Sigma, USA), 0.06% (w / v, ie g / mL) BSA (purchased from Sigma, USA), 0.1% (v / v) Tween-20 (purchased from Sigma, USA), 0.06MpH8.9Tricine (purchased from Merck),
[0060] Specific primers SEQ ID NO: 1-2 or SEQ ID NO: 3-4, 2mM,
[0061] MgCl 2 : Purchased from Sigma, USA,
[0062] 0.2mM dNTPs: purchased from Shanghai Dingguo Biotechnology Co., Ltd.,
[0063] 2U / μL Taq DNA polymerase: purchased from Fermentas, USA.
[0064] (3) Reagent for single-strand purification:
[0065]...
Embodiment 2
[0075] Embodiment 2: detection method.
[0076] Instruments: Bio-Rad S1000 PCR instrument, Beckman Microfuge 22R desktop micro-refrigerated centrifuge, Beijing Liuyi agarose gel electrophoresis instrument, Shanghai Peiqing gel imaging system, QIAGEN PyroMark Q96ID sequencer.
[0077] (1) Extract bacterial DNA, specifically comprising the following steps:
[0078] (1a) 1000 μL of heparin-anticoagulated blood was placed at room temperature for about 10 minutes to precipitate blood cells, and 200 μL was drawn from the interface between serum and blood cells (ie buffy coat), and injected into a 1.5 mL sterilized centrifuge tube.
[0079] (1b) Add 1 mL of 0.87% (w / v, ie g / mL) NH4Cl, shake well, and place in a 37°C metal bath for 15 minutes.
[0080] (1c) Take out, centrifuge at 10000r / min for 5min, and remove the supernatant.
[0081] (1d) Repeat steps (1b) and (1c) 2 times.
[0082] (1e) Add 200 μL of 5% (w / v, ie g / mL) Chelex 100 buffer and 2 μL (20 mg / mL) of proteinase K to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com