New diketone piperazidine-like derivative and application thereof
A technology of diketopiperazines and derivatives, which is applied in the field of medicine, can solve the problems of limited curative effect, increased multidrug resistance and the like, and achieves the effect of strong activity and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
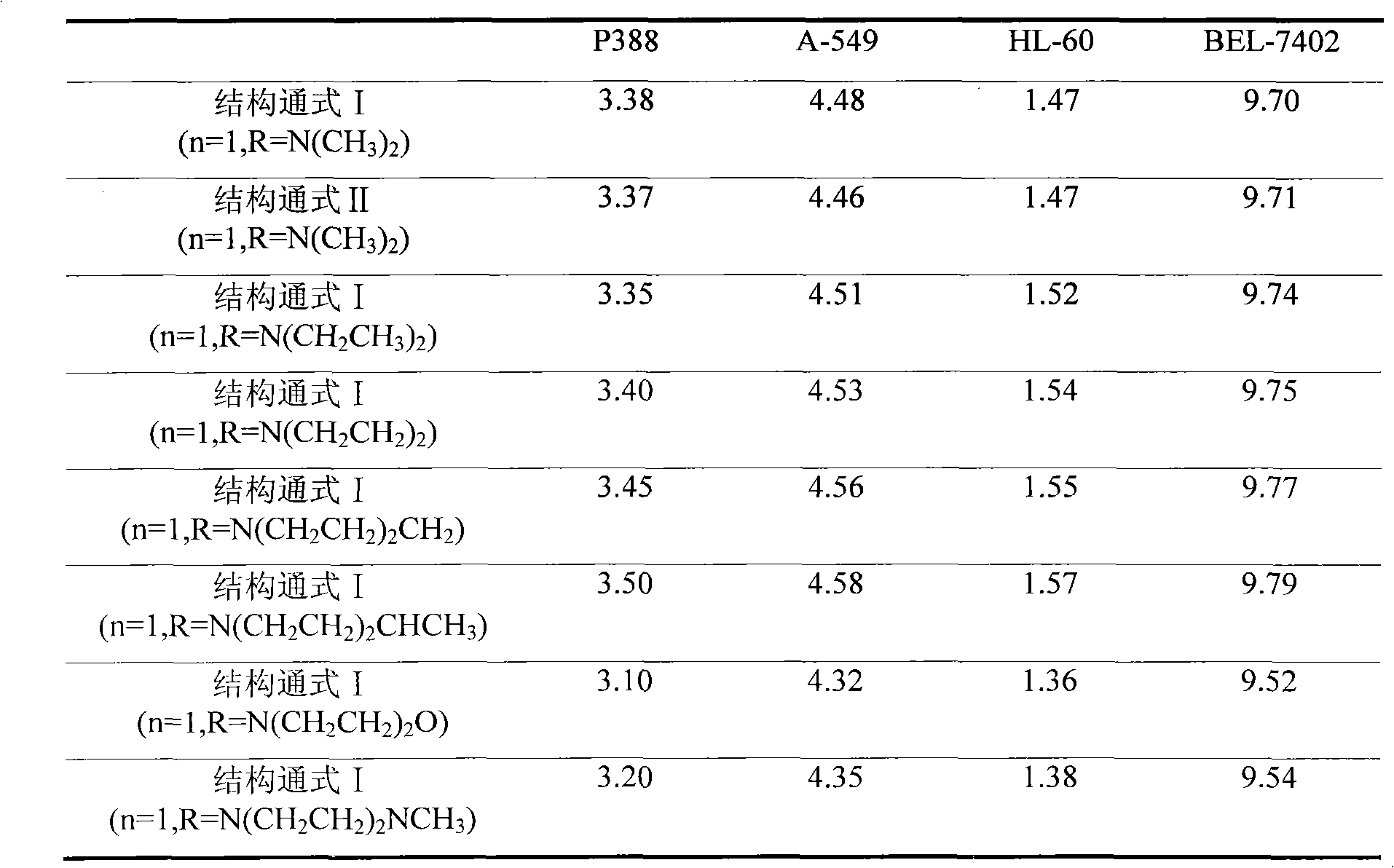
Method used
Image
Examples
example 1
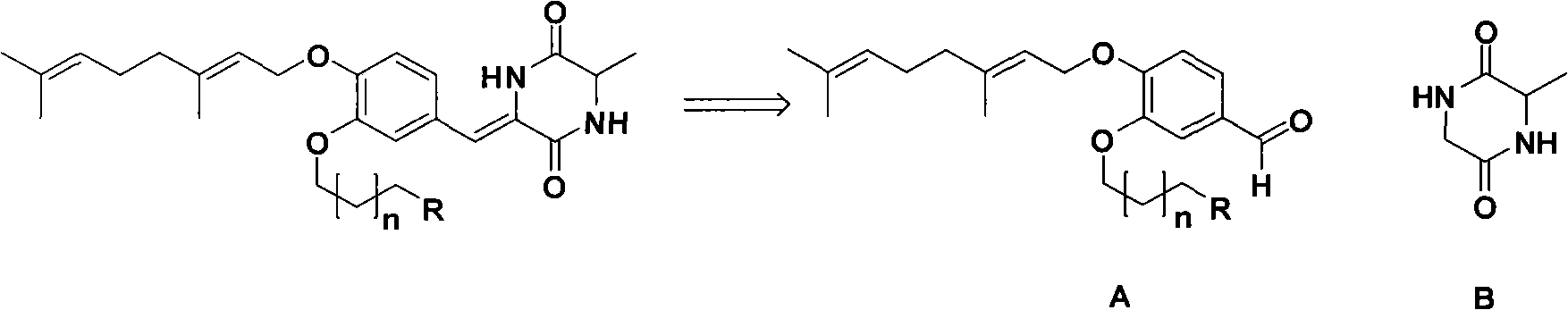
[0016] Total synthesis method: (with n=1 in the general structural formula I series, R=N(CH 3 ) 2 example)
[0017] 1. 3-Hydroxy-4-geraniol benzaldehyde (pre-synthesis 1.0eq), bromochloropropane (1.0eq), triethylamine (1.0eq), dichloromethane as solvent, react in ice-water bath at 0°C for 30min, Silica gel column separation, the product was directly used in the next reaction. The product from the previous step (1.0eq), dimethylamine (1.0eq), triethylamine (cat), and dichloromethane were used as solvents; after mixing, they were stirred at room temperature for 5 hours and separated on a silica gel column to obtain the product (65%).
[0018] 2. Glycine methyl ester hydrochloride (1.0eq), L-alanine (Cbz protected amino group 1.0eq), add DCC (2.0eq), DMAP (cat), dichloromethane as solvent, mix and stir for 4 hours at room temperature , separated on a silica gel column, and the product was directly used in the next reaction. Last step product (1.0eq), H 2 (2.0eq), add ethanol...
example 2
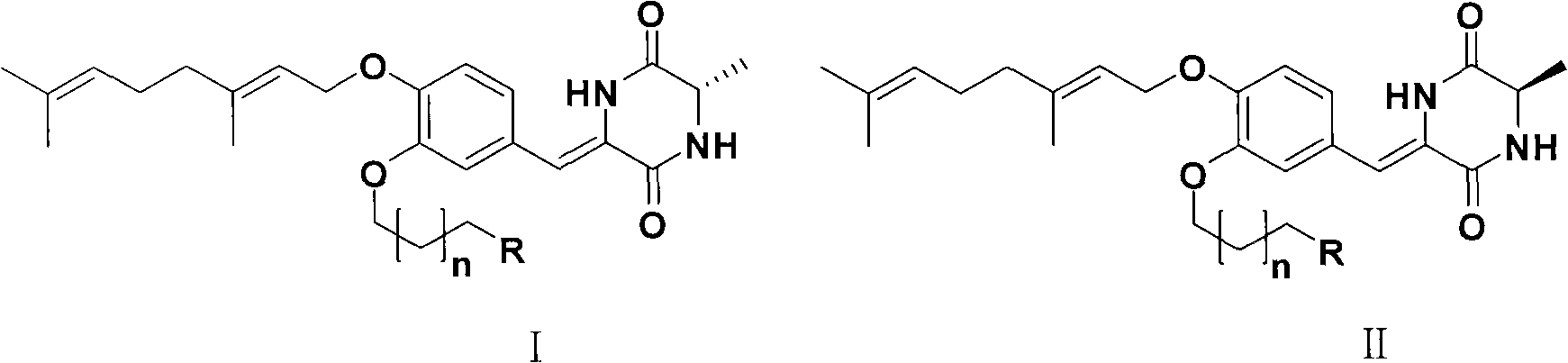
[0022] In the general structural formula I series, n=1, R=N(CH 2 CH 3 ) 2 Prepared by the method 1.2.3 in the above-mentioned Example 1. The NMR data are as follows:
[0023] 1 H-NMR (300MHz, DMSO-d 6 )δ: 9.78 (1H, brs), 8.35 (1H, s), 7.07 (1H, brs), 7.03 (1H, d, J = 8.4Hz), 6.95 (1H, d, J = 8.4Hz), 6.61 ( 1H, s), 5.40 (1H, t, J=6.0Hz), 5.04 (1H, t, J=6.0Hz), 4.54 (2H, d, J=6.0Hz), 4.09 (1H, q, J=6.9 Hz), 4.00(2H, t, J=6.3Hz), 2.51(2H, t, J=6.9Hz), 2.44(4H, q, J=7.2Hz), 2.05(4H, m), 1.78(2H, m), 1.68 (3H, s), 1.62 (3H, s), 1.55 (3H, s), 1.32 (3H, d, J = 6.9 Hz), 0.92 (6H, t, J = 7.2 Hz).
example 3
[0025] In the general structural formula I series, n=1, R=N(CH 2 CH 2 ) 2 Prepared by the method 1.2.3 in the above-mentioned Example 1. The NMR data are as follows:
[0026] 1 H-NMR (300MHz, DMSO-d 6 )δ: 9.78 (1H, brs), 8.35 (1H, s), 7.07 (1H, brs), 7.04 (1H, d, J = 8.4Hz), 6.95 (1H, d, J = 8.4Hz), 6.62 ( 1H, s), 5.40 (1H, t, J=6.0Hz), 5.04 (1H, t, J=6.0Hz), 4.55 (2H, d, J=6.0Hz), 4.10 (1H, q, J=6.9 Hz), 4.00(2H, t, J=6.3Hz), 2.51(2H, t, J=7.2Hz), 2.41(4H, m), 2.05(4H, m), 1.86(2H, m), 1.68( 3H, s), 1.66 (4H, m), 1.62 (3H, s), 1.55 (3H, s), 1.32 (3H, d, J = 6.9 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com