Preparation method of optically pure 4-aryl-2-hydroxy-butyric acid
An aryl and optical technology, which is applied in the field of preparation of optically pure 4-aryl-2-hydroxy-butyric acid, can solve the problems of increased cost, cumbersome operation process, and reduced total yield, and achieves low-cost, repeatable Good sex and high selectivity
Inactive Publication Date: 2011-01-12
SHANGHAI JIAO TONG UNIV
View PDF1 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
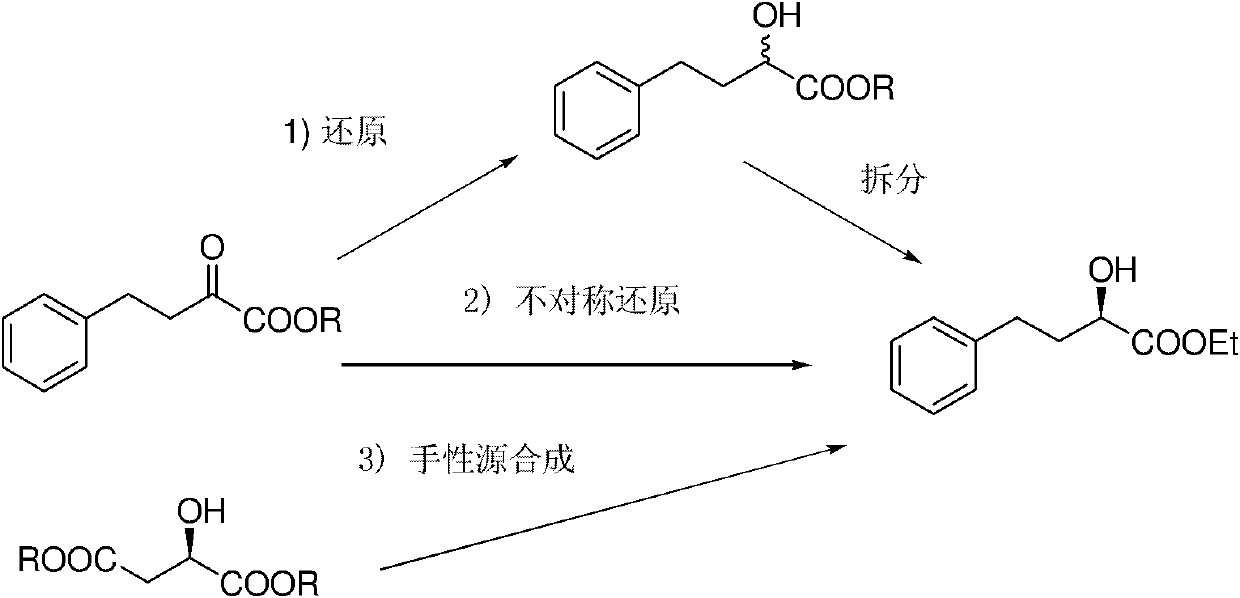
However, this asymmetric reaction needs to first esterify the initial raw material 4-phenyl-2-carbonyl-3-butenoate, and then use a chiral ruthenium catalyst for asymmetric hydrogenation to obtain chiral ethyl butyrate (ee: 94 %), in order to further improve the ee value of the product, the chiral product ethyl butyrate is hydrolyzed into butyric acid, the ee value is increased to more than 99% by recrystallization, and esterified again into optically pure ethyl butyrate, the whole operation process Become cumbersome, increase cost and reduce overall yield (J.Org.Chem.2008, 73, 7209)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
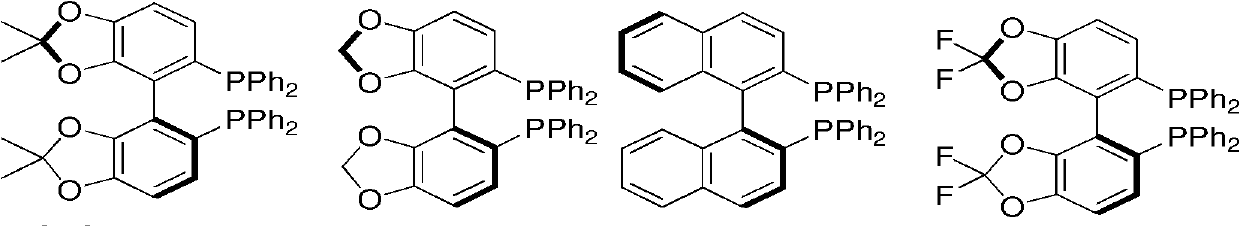
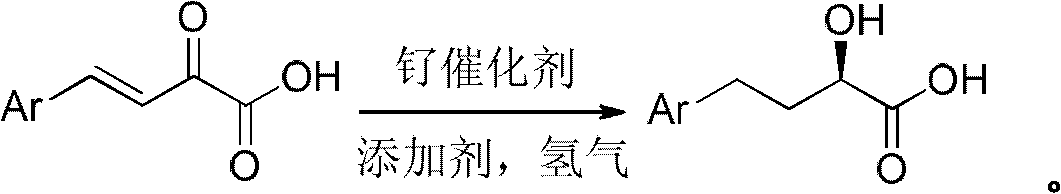
The invention relates to a preparation method of optically pure 4-aryl-2-hydroxy-butyric acid in the technical field of chemical materials. In an organic ether or alcoholic solvent, 4-aryl-2-oxo-3-crotonic acid is catalytically hydrogenated under the coexistence of a ruthenium complex of a united aryl-axis chiral phosphine ligand and an acidic additive to prepare optically pure 4-aryl-2-hydroxy-butyric acid. Starting from the 4-aryl-2-oxo-3-crotonic acid, the invention uses the ruthenium complex of the united aryl-axis chiral phosphine ligand as a catalyst and adds the acidic additive to obtain (R)-4- aryl-2-hydroxy-butyric acid through an asymmetric catalytic hydrogenation. The invention has low cost, stable physical and chemical properties and easy purification and increases an ee value to be larger than 99 percent through a simple and convenient recrystallization method. The reaction formula is carried out as shown in the specification.
Description
technical field The invention relates to a preparation method in the technical field of chemical materials, in particular to a preparation method of optically pure 4-aryl-2-hydroxy-butyric acid. Background technique Optically pure ethyl 4-phenyl-2-hydroxy-butyrate is an important pharmaceutical intermediate and is widely used in the fields of medicine and new materials. Among them, (R)-4-phenyl-2-hydroxy-butyric acid ethyl ester is an important intermediate for the preparation of antihypertensive drugs-pril drugs. Pulil drugs are inhibitors of angiotensin-converting enzyme (ACE), and are a class of antihypertensive drugs that are currently used in a very large amount, accounting for about one-fifth of the entire hypertension drug market. Found through the retrieval of prior art document, the method for preparing (R)-4-phenyl-2-hydroxy-butyric acid ethyl ester is a lot (Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; Wiley-VCH: Weinheim , 20...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C51/367C07C51/36C07C59/48C07C59/64C07C59/56C07D317/60B01J31/24C07B53/00
Inventor 张兆国诸吕锋谢小敏
Owner SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com