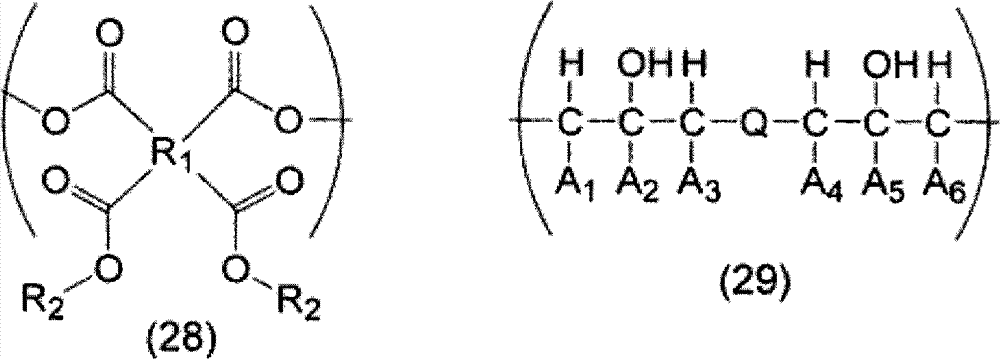Composition for forming resist underlayer film and method for forming resist pattern using the same
A technology of resist underlayer and composition, which is applied in the field of composition and can solve problems such as no record
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
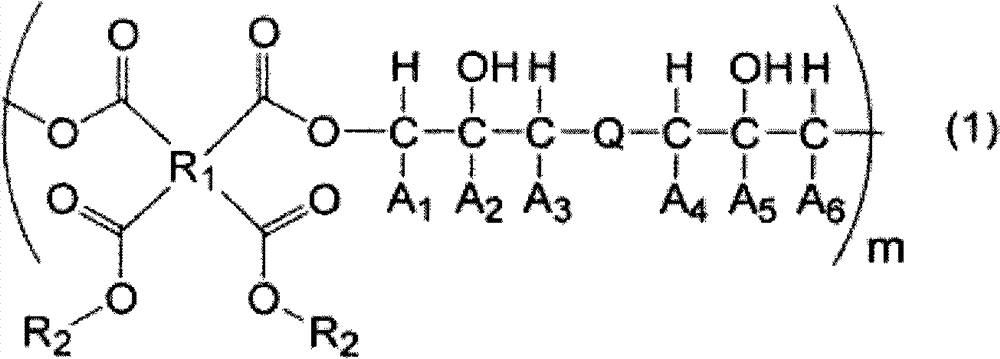
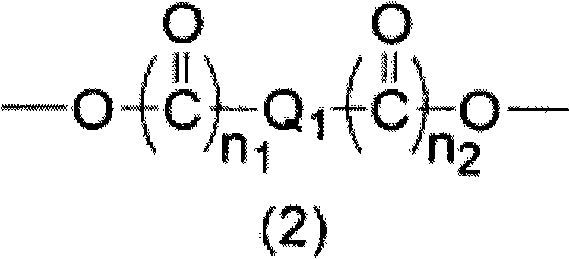
[0107] 9.00 g of monoallyl diglycidyl isocyanuric acid (manufactured by Shikoku Chemical Industry Co., Ltd.) represented by the above formula (9-a), 1,2,3 6.46 g of 4-cyclobutanetetracarboxylic dianhydride and 0.61 g of ethyl triphenylphosphonium bromide were dissolved in 64.29 g of propylene glycol monomethyl ether and reacted at 130°C for 4 hours to obtain a polymer-containing solution . The obtained polymkeric substance is equivalent to containing the polymkeric substance (a: b=1: 1 (molar ratio)) of structural unit shown in above-mentioned formula (13), carries out GPC analysis, the weight average molecular weight of calibration with standard polystyrene as a result is 7,400. 1,2,3,4-Cyclobutanetetracarboxylic dianhydride is obtained, for example, using the method described in JP-A-2-61956 and JP-A-2003-192685.
Synthetic example 2
[0109] 20.00 g of monoallyl diglycidyl isocyanuric acid (manufactured by Shikoku Chemical Industry Co., Ltd.), 7.06 g of 1,2,3,4-cyclobutanetetracarboxylic dianhydride, 2,4-dihydroxy 5.55 g of benzoic acid and 1.34 g of ethyltriphenylphosphonium bromide were dissolved in 79.19 g of propylene glycol monomethyl ether, and reacted at 130° C. for 4 hours to obtain a solution containing a polymer. The obtained polymkeric substance is equivalent to the polymkeric substance (a: b': b "'=2:1:1 (molar ratio)) that contains the structural unit shown in above-mentioned formula (14), carries out GPC analysis, the result is analyzed by standard polymer The styrene-calibrated weight average molecular weight was 8,200.
Synthetic example 3
[0111] Monoallyl diglycidyl isocyanuric acid (manufactured by Shikoku Chemical Industry Co., Ltd.) 8.00 g, 1,2,3,4-cyclopentanetetracarboxylic acid dicarboxylic acid represented by the above formula (7-d) After dissolving 6.04 g of anhydride (manufactured by Tokyo Chemical Industry Co., Ltd.) and 0.53 g of ethyltriphenylphosphonium bromide in 34.01 g of propylene glycol monomethyl ether, they were reacted at 130° C. for 4 hours to obtain a polymer-containing solution. The obtained polymkeric substance is equivalent to the polymkeric substance (a:b=1:1 (molar ratio)) that contains the structural unit shown in above-mentioned formula (15), carries out GPC analysis, the weight average molecular weight of result calibration with standard polystyrene for 10,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com