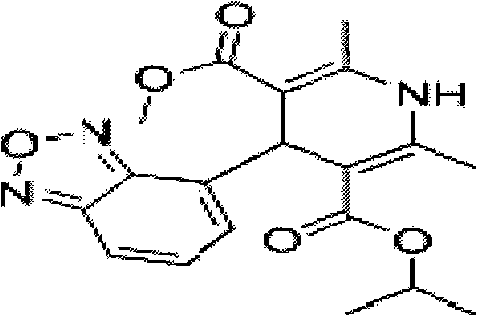
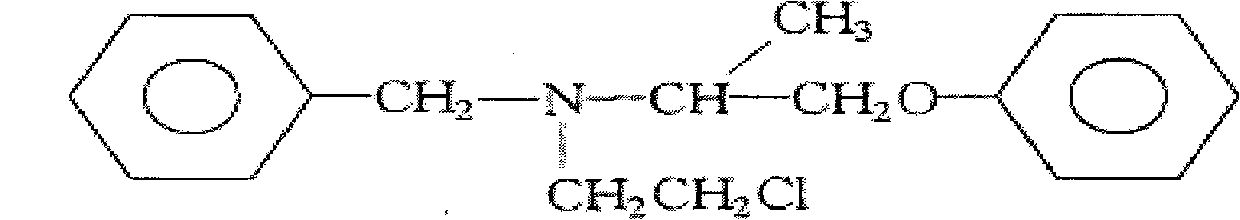Ointment for treating high blood pressure and preparation method thereof
An ointment and active technology, applied in the field of ointment for the treatment of high blood pressure and its preparation, can solve the problems that the effect needs to be improved, and achieve the effects of reducing the limitation of inability to use drugs, fast absorption, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The ointment of the present embodiment is prepared from the following components by weight percentage:
[0053] isradipine 2%, urapidil 5%, clonidine 1%, phenoxybenzamine 2%; and include sorbitol, glycerol, parabens, di-tert-butyl-p-cresol, tert-butyl Base 90% including Hydroxyanisole, Gallic Acid, Xanthan Gum, Locust Bean Gum, Gum Arabic, Menthol, Dimethyl Sulfoxide and Laurethrone.
[0054] Wherein the base material volume ratio is:
[0055] Sorbitol 0.2%, Glycerin 0.03%, Parabens 0.75%, Di-tert-butyl-p-cresol 0.036%, tert-butylhydroxyanisole 0.05%, Gallic acid 0.01%, Xanthan gum 0.5% , locust bean gum 0.19%, gum arabic 0.027%, menthol 0.005%, dimethyl sulfoxide 0.32% and lauroazepam 0.15%.
[0056] The preparation method of ointment, comprises the steps:
[0057] 1) Mix the base materials in terms of weight percentage, heat and stir at 60°C to dissolve;
[0058] 2) After the active compound is ground and sieved, it is added to the mixture obtained in step 1) and ...
Embodiment 2
[0060] The ointment of the present embodiment is prepared from the following components by weight percentage:
[0061] Isradipine 20%, Urapidil 13%, Clonidine 10%, Phenoxybenzamine 17%, and include propylene glycol, polyethylene glycol, glycerin, sodium pyrrolidone carboxylate, sorbitol, glycerol, parabens Esters, Sodium Benzoate, Citric Acid, Di-tert-Butyl-p-Cresol, tert-Butyl Hydroxyanisole, Gallic Acid, Propyl Gallate, Sodium Sorbate, Tocopherol, Xanthan Gum, Guar Gum, Capita Gum, locust bean gum, gum arabic, menthol, dimethyl sulfoxide and lauroazepam base 40%.
[0062] Wherein said base material volume ratio is:
[0063] Propylene Glycol 0.005%, Polyethylene Glycol 0.03%, Glycerin 0.07%, Sodium Pyrrolidone Carboxylate 0.0065%, Sorbitol 0.35%, Glycerin 0.57%, Parabens 0.15%, Sodium Benzoate 0.85%, Citric Acid 0.054 %, di-tert-butyl-p-cresol 0.025%, tert-butyl hydroxyanisole 0.035%, gallic acid 0.05%, propyl gallate 0.055%, sodium sorbate 0.15%, tocopherol 0.45%, xanthan ...
Embodiment 3
[0068] The ointment of the present embodiment is prepared from following components by weight percentage:
[0069] Isradipine 5%, Urapidil 7%, Clonidine 3%, Phenoxybenzamine 5%; and including propylene glycol, polyethylene glycol, sorbitol, glycerol, parabens, citric acid, Base consisting of di-tert-butyl-p-cresol, propyl gallate, sodium sorbate, tocopherol, xanthan gum, locust bean gum, gum arabic, menthol, dimethyl sulfoxide and laurocaprone 70% .
[0070] Wherein said base material volume ratio is:
[0071] Propylene glycol 0.11%, polyethylene glycol 0.17%, sorbitol 0.21%, glycerin 0.061%, parabens 0.05%, citric acid 0.03%; di-tert-butyl p-cresol 0.02%, propyl gallate 0.12%, Sodium Sorbate 0.19%, Tocopherol 0.21%, Xanthan Gum 0.1%, Locust Bean Gum and Gum Arabic 0.06%, Menthol 0.071%, Dimethyl Sulfoxide 0.06%, and Laurocaprone 0.28%.
[0072] The preparation method of ointment, comprises the steps:
[0073] 1) Mix the base materials in the weight percentage, heat and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com