Method for preparing porcine parvovirus inactivated vaccines
A technology for parvoviruses and inactivated vaccines, applied in biochemical equipment and methods, antiviral agents, viruses/bacteriophages, etc., can solve the problems of high labor intensity, hidden pollution, large difference between vaccine batches, etc., and improve ST Effects of cell density, increased virus titer, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Screening of suitable cell lines
[0024] The porcine parvovirus BJ-2 strain was inoculated in ST or IBRS-2 subculture cells, in DMEM medium, the culture conditions were controlled at pH 7.0-7.3, temperature 35-37°C, subcultured continuously, so that the virus adapted to the cells, and determined Virus reproduction titer, to reach the required virus content requirement ≥ 10 6.0 TCID 50 / ml to screen out suitable cell lines.
[0025] A suitable cell line should have the characteristics of good shape, stable and consistent state, and high toxin production. The specific cell standards are:
[0026] Cell morphology: use DMEM medium containing 10% fetal bovine serum in 5% CO 2 Incubate at 37°C in an incubator for 6 hours to adhere to the wall, and grow into a single layer after 40 hours. Observed under a microscope, the cells were irregular in shape.
[0027] Exogenous virus inspection: Inspect according to the appendix of the current "Chinese Veterinary Phar...
Embodiment 2
[0035] Embodiment 2 virus identification
[0036] Make BJ-2 strain virus on the suitable cell line screened out in embodiment 1, add in the DMEM medium that has 5~10% serum concentration, control culture condition is pH value 7.0~7.3, temperature 35~37 ℃ , continue to subculture, and at the same time identify the specificity, immunogenicity, virulence and other virus characteristics of the virus, and conduct gene sequence analysis to ensure that the virus is different from the original isolate in the specificity, virulence, immunogenicity and other aspects during the culture process. No significant changes have occurred.
[0037] (1) Specific identification of virus
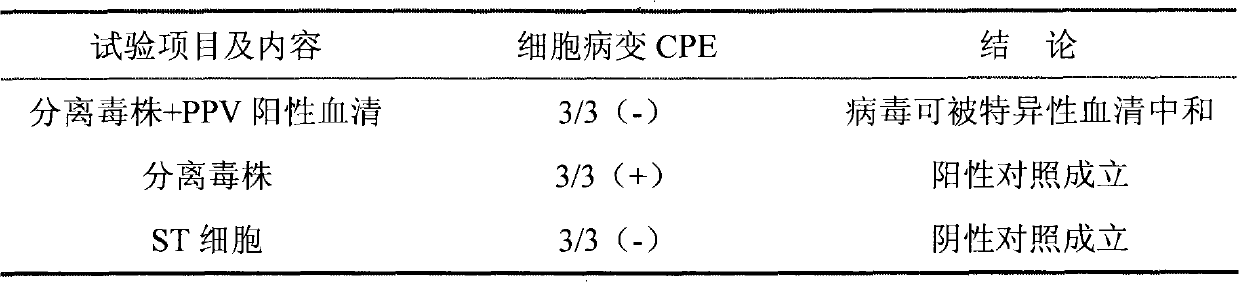
[0038] Physical and chemical properties testing: the results show that the isolate is insensitive to lipid solvents, acids, and alkalis, and has strong thermal stability. These characteristics are consistent with the general characteristics of PPV.
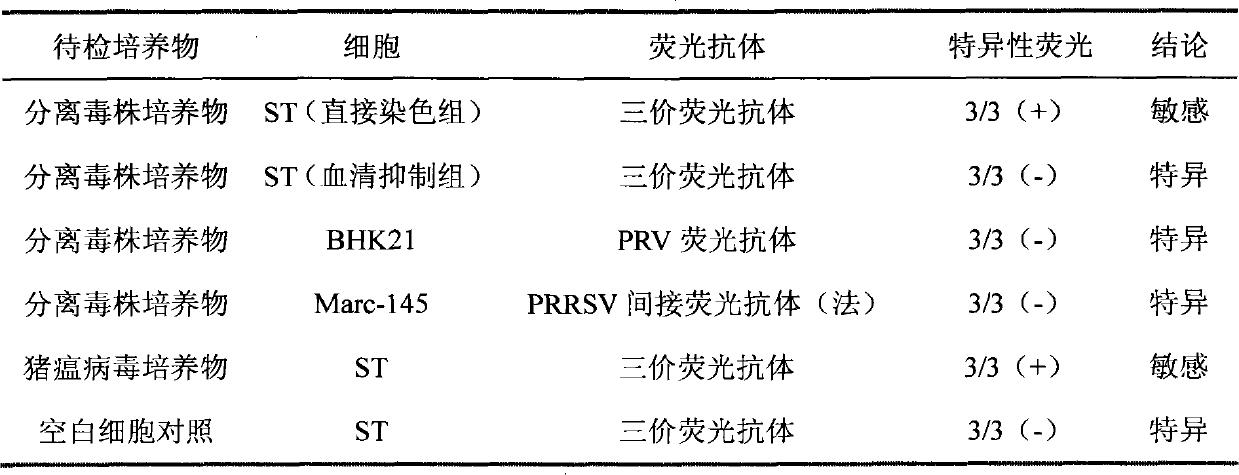
[0039] Fluorescence inhibition test: Porcine parvovirus (PPV...
Embodiment 3
[0055] Example 3 Cell Line Culture
[0056] Use a square bottle to culture the selected ST cells or IBRS-2 cells recovered from liquid nitrogen. The culture conditions include: pH value 7.0-7.3, temperature 35-37°C, DMEM medium; culture for 48-72 hours, culture until the cells The concentration is 3~6×10 5 cells / ml, you can;
[0057] The cell line was cultured with microcarriers in a bioreactor, and the culture conditions were as follows: the medium was DMEM, and the cell seeding density was 1-2×10 5 cells / ml, the microcarrier concentration is 3-5g / L, the dissolved oxygen is 30%-60%, the pH value is 7.0-7.3, the temperature is 35-37°C, the rotation speed is 40-60r / min, and the cell concentration is 3-5 ×10 6 cells / ml. Wherein, the microcarrier is a spherical carrier Cytodex1 or a sheet carrier.
[0058] According to the growth characteristics of the cells and the size of the bioreactor volume, add fresh DMEM medium at a flow rate of 2 to 3 bioreactor volumes / day, and pump...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com