Method for converting trans-vinylcyclopropane to cis-vinylcyclopropane
A vinylcyclopropane and basecyclopropane technology, which is applied in the field of conversion of trans-vinylcyclopropane to cis-vinylcyclopropane, can solve the problem that chiral catalysts or optically pure raw materials are expensive, limited in application, and rare in starting materials. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
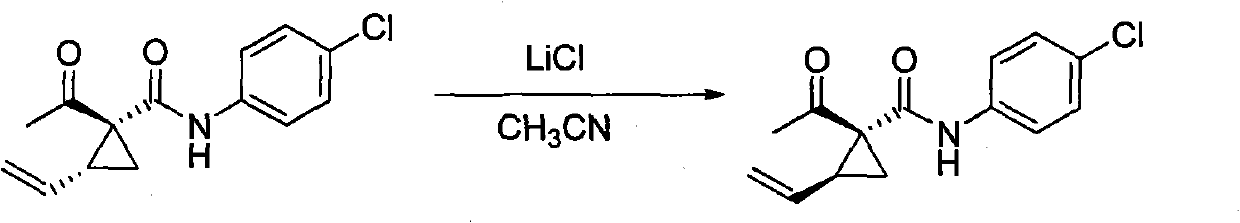
[0023] 1 H NMR determination of the trans-vinylcyclopropane / cis-vinyl ring in the synthesized 2-vinyl-1-acetyl-N-(4-chlorophenyl)-1-amidocyclopropane stereoisomer mixture Propane=5; Add acetonitrile (5.0 ml) and this vinylcyclopropane stereoisomer mixture (0.527 g, 2.0 mmol) and lithium chloride (0.254 g, 6.0 mmol) in a 25 ml round bottom flask; room temperature Stir for 10 hours; the reaction solution is poured into 30 milliliters of water, suction filtered, and air-dried to obtain 0.519 grams of solid, which is subjected to 1 H NMR detection, cis-vinylcyclopropane / trans-vinylcyclopropane=8.5; separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain white solid cis-2-ethylene Base-1-acetyl-N-(4-chlorophenyl)-1-amidocyclopropane 0.464 gram, trans-vinylcyclopropane is transformed into cis-vinylcyclopropane and the transformation efficiency is 87%; Conversion is as follows :
[0024]
Embodiment 2
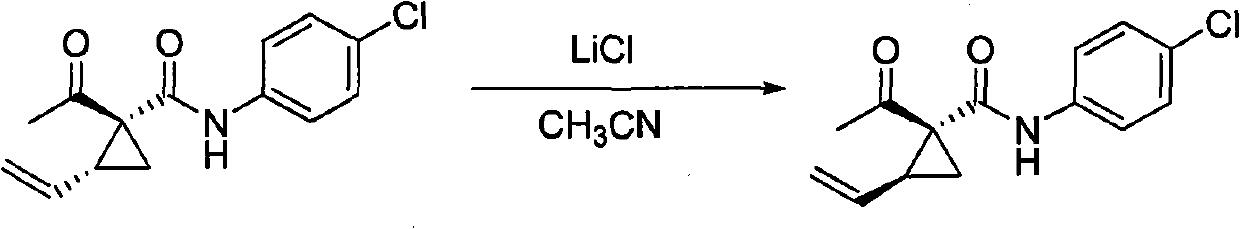
[0026] 1 H NMR technique identified trans-vinylcyclopropane / cis-vinyl in the synthesized 2-vinyl-1-acetyl-N-(2-methylphenyl)-1-amidocyclopropane stereoisomer mixture Cyclopropane=6; Acetonitrile (5.0 mL) and this vinylcyclopropane stereoisomer mixture (0.487 g, 2.0 mmol) and lithium chloride (0.254 g, 6.0 mmol) were added to a 25 mL round bottom flask; Stir at room temperature for 10 hours; the reaction solution was poured into 30 ml of water, suction filtered, and air-dried to obtain 0.479 g of solid, which was subjected to 1H NMR detection, cis-vinylcyclopropane / trans-vinylcyclopropane=8.7; separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain white solid cis-2-ethylene 0.430 g of yl-1-acetyl-N-(2-methylphenyl)-1-amidocyclopropane, the conversion rate of trans-vinylcyclopropane to cis-vinylcyclopropane was 87%; the conversion was as follows Mode:
[0027]
Embodiment 3
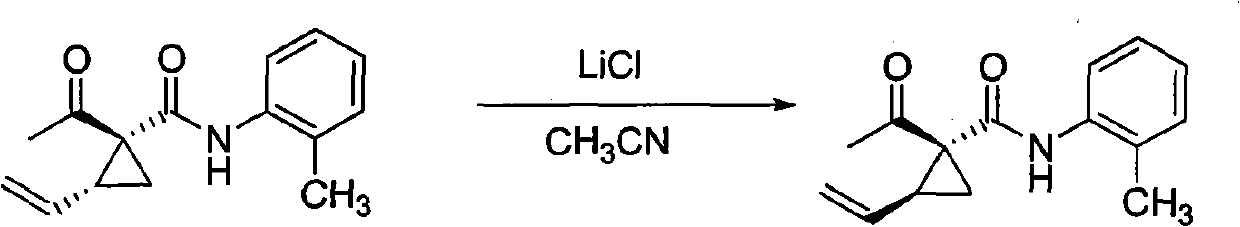
[0029] 1 H NMR technique determination of trans-vinylcyclopropane / cis-ethylene in the synthesized mixture of 2-vinyl-1-acetyl-N-(4-methoxyphenyl)-1-amidocyclopropane stereoisomers Cyclopropane=6.5; Add acetonitrile (5.0 milliliters) and this vinyl cyclopropane stereoisomer mixture (0.519 gram, 2.0 mmol) and lithium chloride (0.254 gram, 6.0 mmol) in 25 milliliters of round bottom flasks Stir at room temperature for 10 hours; The reaction solution was poured into 30 milliliters of water, suction filtered, and air-dried to obtain 0.511 grams of solids, which were subjected to 1 H NMR detection, cis-vinylcyclopropane / trans-vinylcyclopropane=8.2; separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain white solid cis-2-ethylene Base-1-acetyl-N-(4-methoxyphenyl)-1-amidocyclopropane 0.455 grams, trans-vinylcyclopropane to cis-vinylcyclopropane conversion conversion rate of 86%; conversion as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com