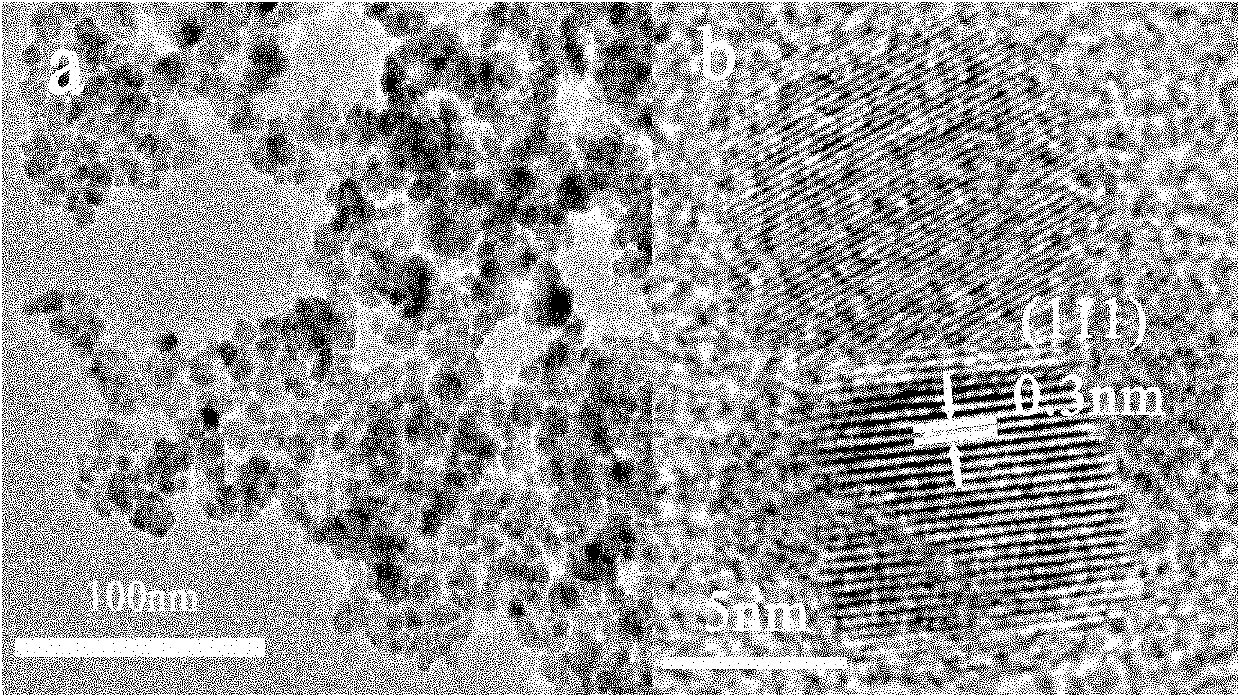
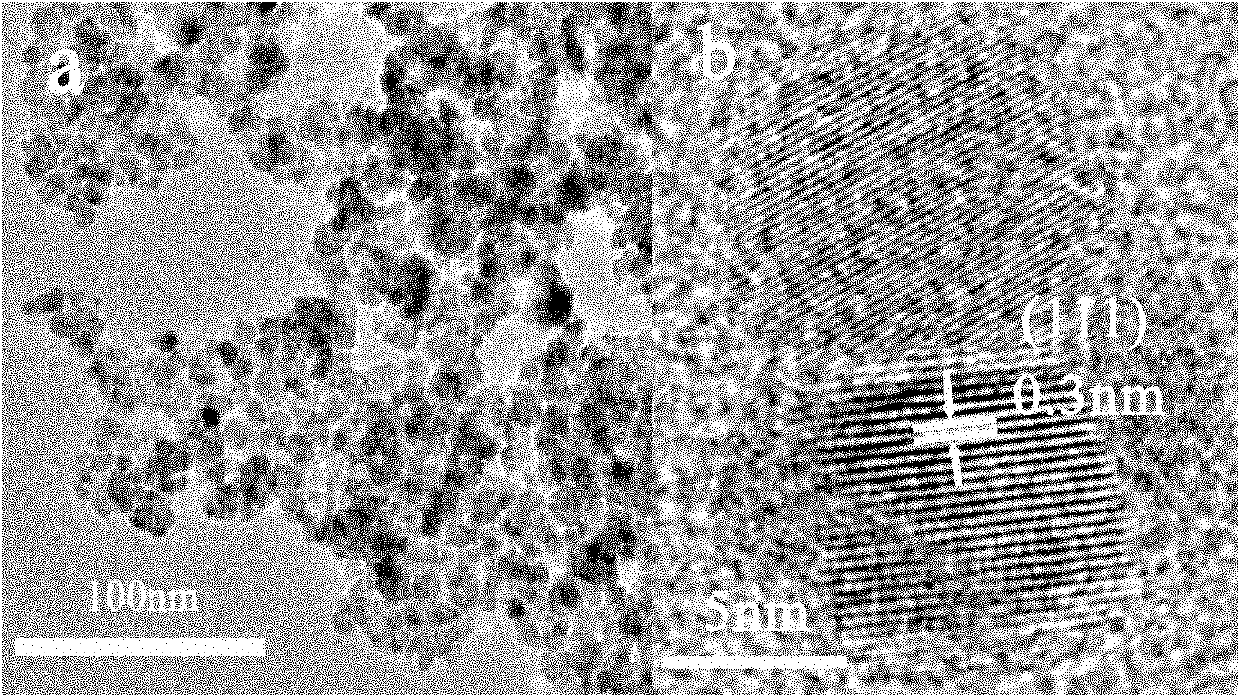
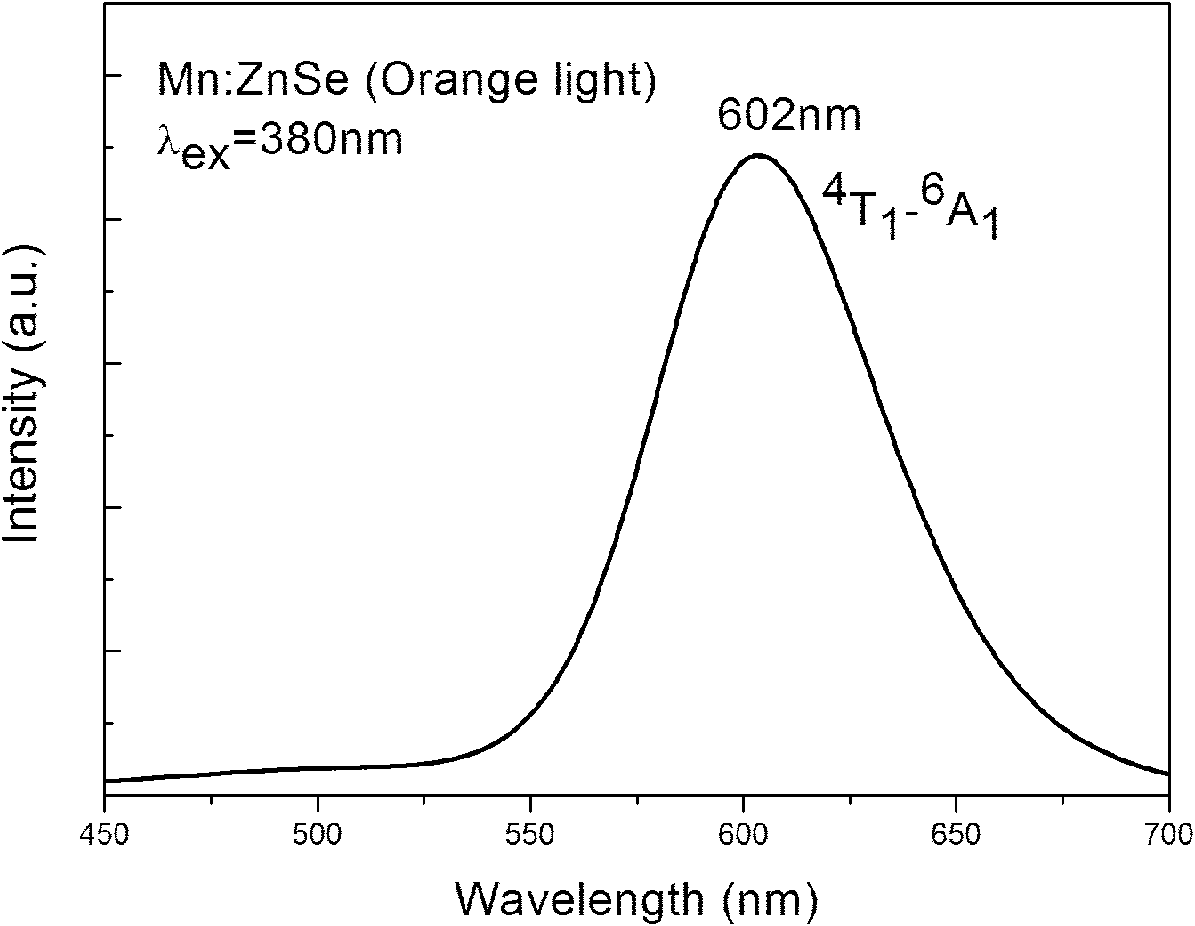
Method for aqueous phase synthesis of manganese-doped zinc selenide adjustable-colour fluorescent quantum dot
A technology of fluorescent quantum dots and manganese doping, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of energy loss, no adjustability of fluorescence spectrum, low quantum yield, etc., and achieve simple production equipment and low energy consumption The effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under nitrogen protection atmosphere, 0.0247g selenium powder and 0.0299gNaHB 4 Dissolve in 10mL high-purity water to prepare NaHSe solution for later use. 1.064g MPA was dissolved in high-purity water, then the pH of the above solution was adjusted to 11.0 with 2.0mol / L NaOH solution and the volume was fixed to 10mL, and the prepared stabilizer solution was placed in a fume hood for subsequent use. 0.0495g MnCl 2 4H 2 O was dissolved in 10 mL of high-purity water to prepare Mn 2+ The solution is ready for use. 0.1372g Zn(OAc) 2 2H 2 O was dissolved in 25mL high-purity water to prepare Zn 2+ The solution is ready for use.
[0036] 1mL Mn 2+ The solution was placed in a 50mL three-necked flask, passed through nitrogen for 30min, and then heated to 100°C with an oil bath to reflux. The newly prepared NaHSe solution was quickly injected into the above solution and refluxed for 40 minutes to obtain MnSe nanocrystal nuclei; the reaction solution was cooled to 80°C, ...
Embodiment 2
[0042] Under nitrogen protective atmosphere, 0.0247g selenium powder and 0.0449gNaHB 4 Dissolve in 10mL high-purity water to prepare NaHSe solution for later use. 0.3546g MPA was dissolved in high-purity water, then the pH of the above solution was adjusted to 12.0 with 2.0mol / L NaOH solution and the volume was fixed to 10mL, and placed in a fume hood for later use. 0.0495g MnCl 2 4H 2 O was dissolved in 10 mL of high-purity water to prepare Mn 2+ The solution is ready for use. 0.2058g Zn(OAc) 2 2H 2 O was dissolved in 25mL high-purity water to prepare Zn 2+ The solution is ready for use.
[0043] 1mL Mn 2+ The solution was placed in a 50mL three-necked flask, passed through nitrogen for 30min, and then heated to 100°C with an oil bath to reflux. The newly prepared NaHSe solution was quickly injected into the above solution and refluxed for 20 minutes to obtain MnSe nano crystal nucleus. The reaction solution was cooled to 70°C, 1mL Zn(OAc) 2 Add it into the flask w...
Embodiment 3
[0045] Under nitrogen protection atmosphere, 0.0371g selenium powder and 0.0598gNaHB 4 Dissolve in 10mL high-purity water to prepare NaHSe precursor solution for later use. 1.596g MPA was dissolved in high-purity water, then the pH of the above solution was adjusted to 13.0 with 2.0mol / L NaOH solution and the volume was fixed to 10mL, and placed in a fume hood for later use. 0.0495g MnCl 2 4H 2 O was dissolved in 10 mL of high-purity water to prepare Mn 2+ The solution is ready for use. 0.2744g Zn(OAc) 2 2H 2 O was dissolved in 25mL high-purity water to prepare Zn 2+ The solution is ready for use.
[0046] 1mL Mn 2+ The precursor solution was placed in a 50mL three-necked flask, nitrogen gas was passed for 30min, and then the temperature was raised to 90°C with an oil bath to reflux. The newly prepared NaHSe solution was quickly injected into the above solution and refluxed for 60 minutes to obtain MnSe nano crystal nucleus. The reaction solution was cooled to 60°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com