Nano-scale contrast agents and methods of use
A technology of contrast enhancers, objects, applied in the direction of applications, instruments for radiodiagnosis, general/multifunctional contrast agents, etc., which can solve the problems of lack, lack of clinical tools, limited development and effectiveness of nanosystems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 - Preparation and Characterization of Schematic Nanoprobes
[0044] High-concentration iodine (600 mgl / mL) was prepared by dissolving iodixanol powder (Visapaque 320 lyophilized from GE Healthcare) into ultrapure water under continuous stirring and heating at 70°C. The liposome alcohol solution was hydrated with iodine solution at 70°C, and the liposome alcohol solution contained 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol in a molar ratio of 55:40:5 and 1,2-dipalmitoyl-sn-glycerol-3-phosphoethanolamine-N-[methoxy(poly(ethylene glycol))-2000] (Mpeg2000-DSPE), and then extruded in a LipexThermoline extruder (Northern Lipids , Vancouver, Canada) on sequential extrusion. This results in the encapsulation of the iodine solution within the central aqueous core of PEGylated liposomes. Free unencapsulated iodixanol was replaced by two days of dialysis against 300 mM NaCl using a 100,000 MWCO dialysis tubing using a saline solution (300 mM NaCl...
Embodiment 2
[0045] Example 2 - Imaging studies using the nanoprobes of Example 1
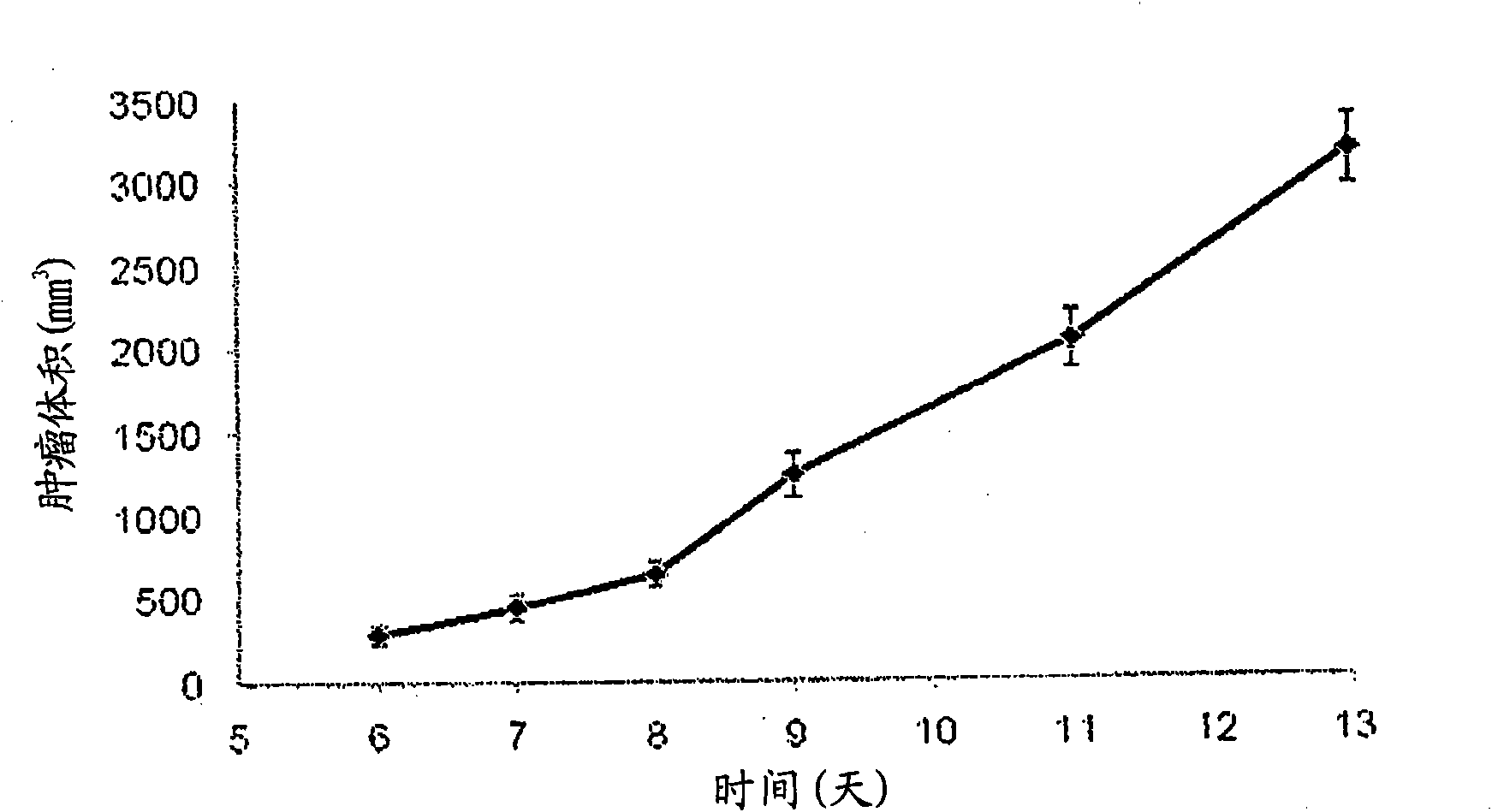
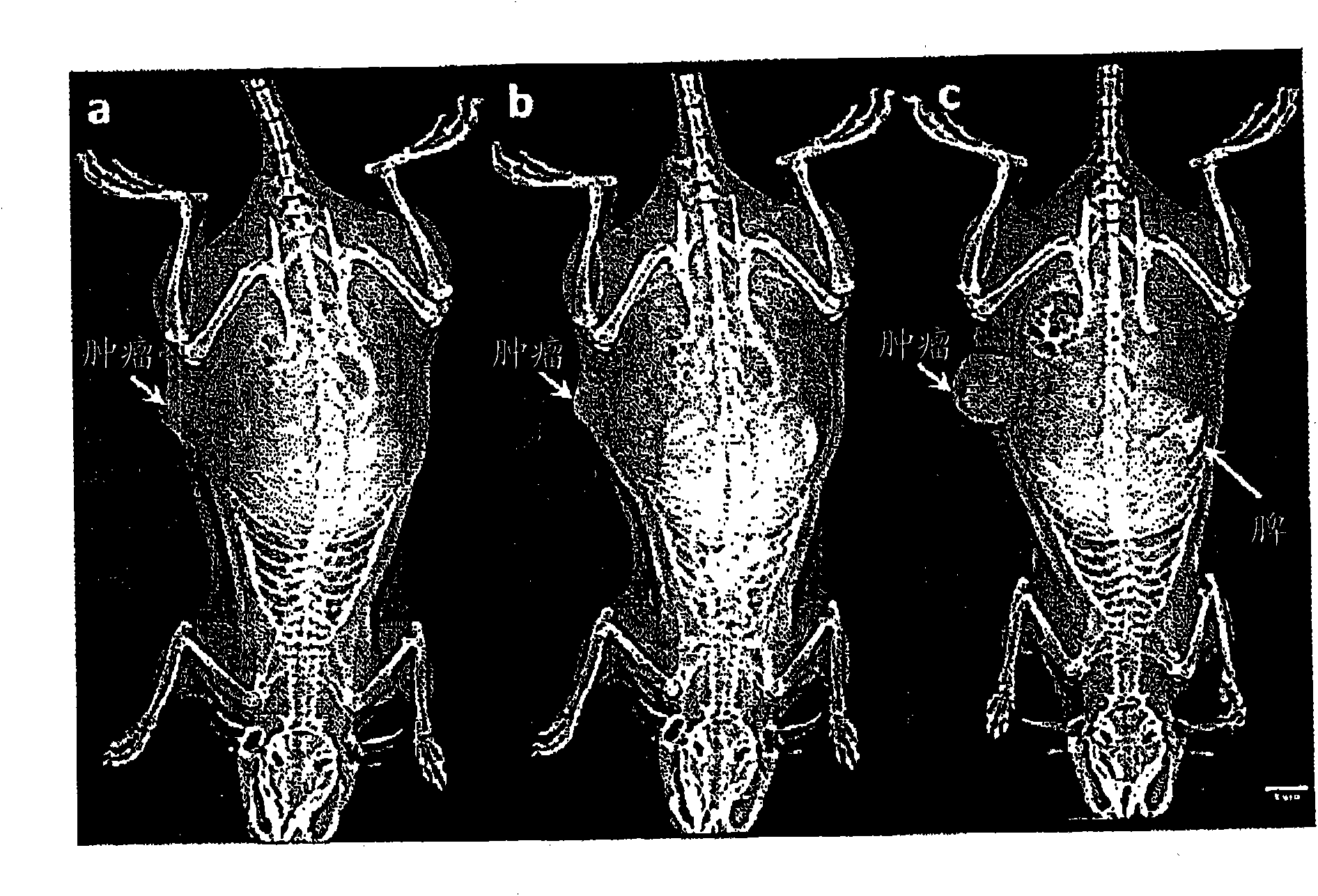
[0046] The nanoprobe of Example 1 was tested in a rat thoracic tumor model formed by transplanting breast cancer cells (13762MAT BIII from ATCC) into the right flank of female Fisher rats. Imaging studies started seven days after tumor implantation (tumor volume approximately 440mm 3 , see figure 1 tumor growth curve). Tumor volumes (n=15) were obtained by calipers.
[0047] Contrast-enhanced mammography was performed using a commercial digital mammography system (Senographe 2000D, GE Healthcare) at 49 kVp and 63 mAs with a rhodium target and an additional copper filter of 0.3 mm thickness. These settings are used to shape the X-rays to have an energy optimized for iodine. Under these conditions, an optimized X-ray spectrum is obtained that contains the maximum number of X-rays with energy on the k-edge of iodine, while at the same time significantly reducing the X-ray dose compared to standard mammo...
Embodiment 3
[0051] Embodiment 3-comparative embodiment (control group)
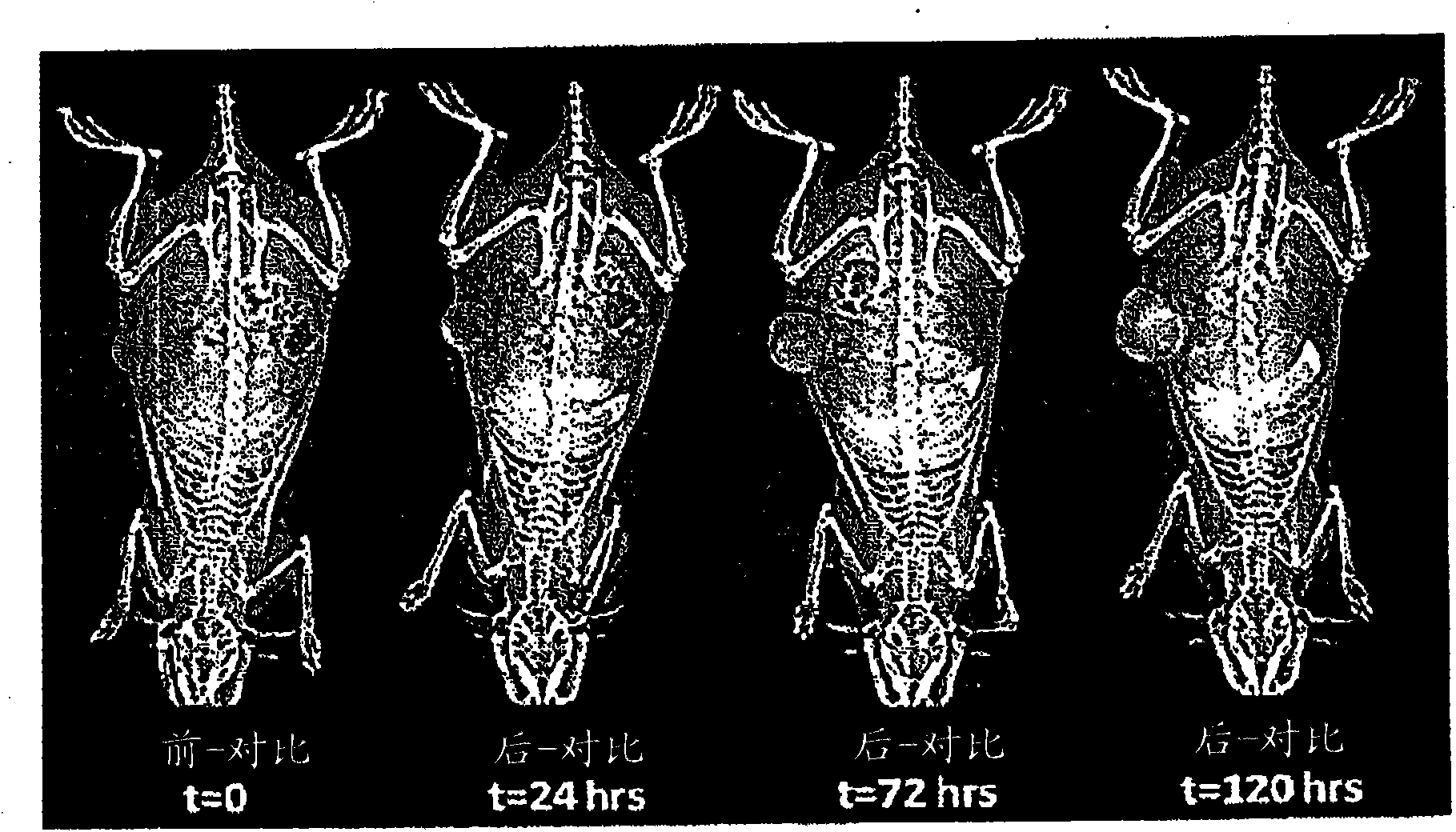
[0052] Figure 6 Whole body mammography of rats not injected with contrast agent (control group) is shown. Another control group was injected with a conventional iodinating agent (iohexol) at an iodine dose equivalent to 1,344 mgl / mL of high-dose nanoprobes. (See Figure 7 ). General vasculature and tumor lesions showed slight enhancement within the first minute after injection, but the iodinated reagent was rapidly cleared via the kidneys. (See Figure 8 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com