Method for detecting quality of Chinese medicinal composition for treating proliferation of mammary gland
A technology of mammary gland hyperplasia and traditional Chinese medicine granules, which is applied in drug combinations, medical formulas, measuring devices, etc., to achieve the effects of precise drug efficacy, guaranteed quality, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Prescription: Hawthorn 1200g, Fried Malt 2000g, Mildew Spatholobus 2000g, Tongcao 660g.
[0033] 2. Preparation method: decoct the above four medicinal materials with water three times, each time for 0.5 hours, combine the decoction, filter, and concentrate the filtrate to a thick paste with a relative density of 1.10-1.12 (70°C), add an appropriate amount of dextrin, and flow Bed granulation, drying, sizing, and granulation to obtain the traditional Chinese medicine granules of the present invention.
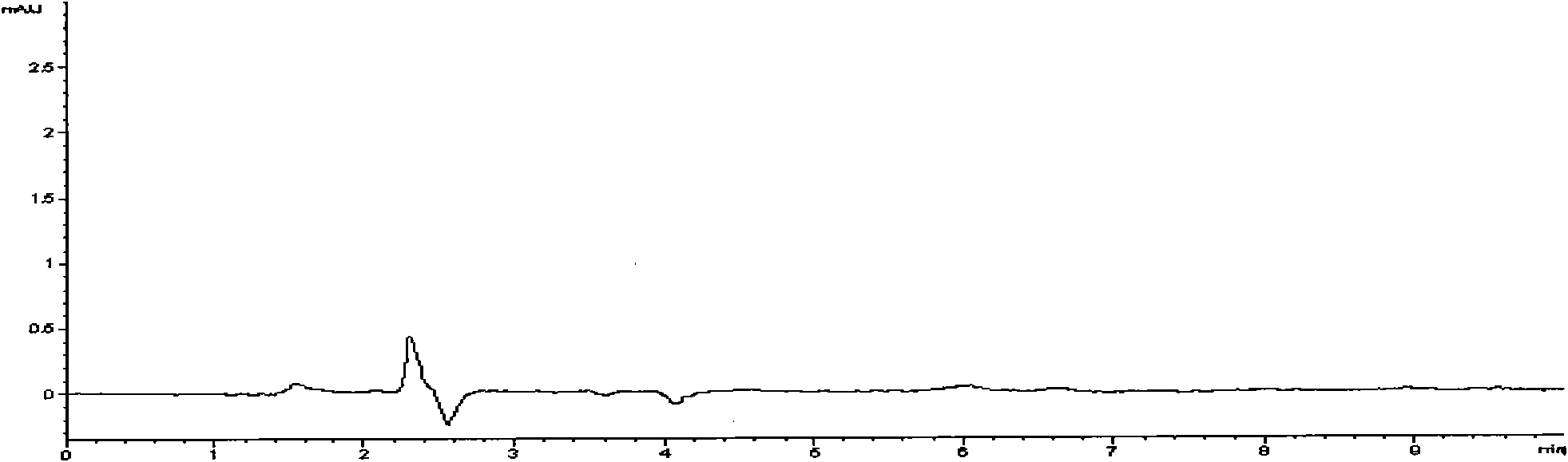
[0034] 3. The identification and content determination methods are as follows:
[0035] 1. The identification method includes the following steps:
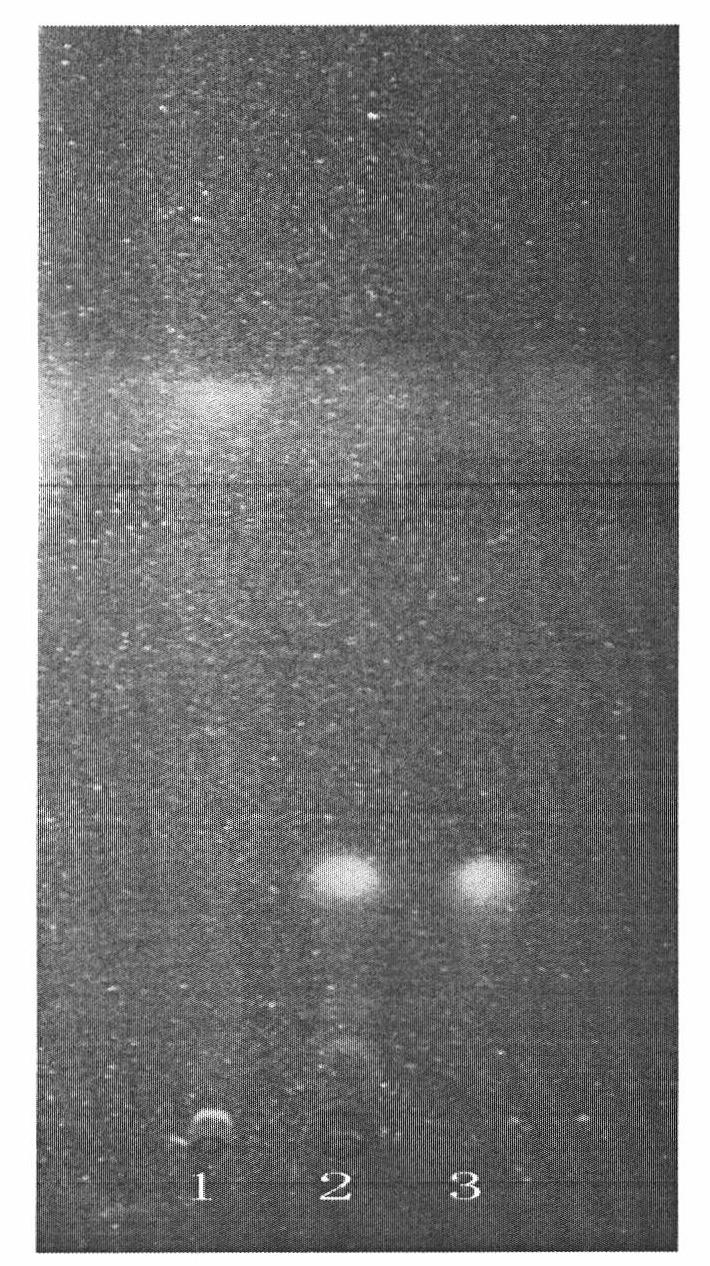
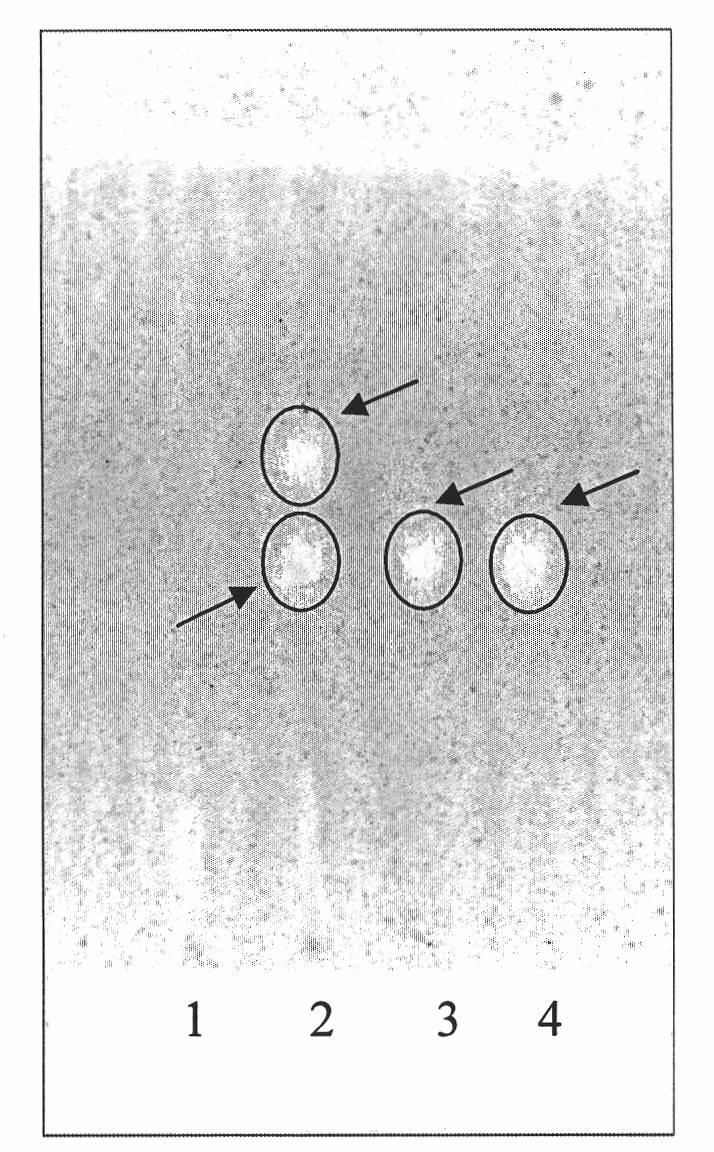
[0036] (1) Specificity test for the identification of Spatholobus Spatholobus
[0037] Preparation of the test solution: Take 10g of the Chinese medicine granule sample, add 50ml of water to dissolve it, add 100ml of ether for ultrasonic treatment for 30min, separate the ether layer, evaporate to dryness, add 1ml of met...
Embodiment 2
[0057] Get the Chinese medicine granule of embodiment 1, identification method comprises the steps:
[0058] (1) Identification of Spathiphyllum Spatholobus: Take 15g of sample, add 50ml of water to dissolve it, add 100ml of ether for ultrasonic treatment (power 320W, frequency 40kHz) for 20min, separate the ether layer, evaporate to dryness, add 1ml of methanol to dissolve the residue, and use it as the test sample solution. Another formononetin reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. Test according to thin-layer chromatography (Appendix VIB of "Chinese Pharmacopoeia" in 2005), take 1 μl of the reference substance solution and 16 μl of the test solution, respectively spot on the same silica gel G thin-layer plate, and mix with cyclohexane-ethyl acetate Ester-chloroform (20:12:3) was used as the developer, developed, taken out, dried in the air, and inspected under a UV lamp at 254nm. In the chromatogr...
Embodiment 3
[0068] Get the pharmaceutical composition among the embodiment 1, identification method comprises the steps:
[0069] (1) Identification of Spathiphyllum Spatholobus: take 8g of sample, add 50ml of water to dissolve it, add 100ml of ether for ultrasonic treatment (power 320W, frequency 40kHz) for 40min, separate the ether layer, evaporate to dryness, add 1ml of methanol to dissolve the residue, and use it as the test sample solution. Another formononetin reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. Test according to thin-layer chromatography (Appendix VIB of "Chinese Pharmacopoeia" in 2005), take 3 μl of the reference substance solution and 25 μl of the test solution, respectively spot on the same silica gel G thin-layer plate, and mix with cyclohexane-ethyl acetate Ester-chloroform (20:12:3) was used as the developer, developed, taken out, dried in the air, and inspected under a UV lamp at 254nm. In the ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com