Method for producing recombinant high-activity manganese superoxide mutase
A superoxide and dismutase technology, applied in the biological field, can solve problems such as low activity and incorrect folding process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the soluble expression of recombinant human Mn-SOD
[0054] Design the following primers:
[0055] Forward: 5-GACATATGAAGCACAGCCTCCCCGACC-3' (SEQ ID NO: 1);
[0056] Reverse: 5'-GCAAGCTTGCATAACGATCGTGGTTTAC-3' (SEQ ID NO: 2).
[0057] Using human placenta cDNA (purchased from Invitrogen) as a template, PCR amplification was performed with the aforementioned primers to obtain the coding sequence of human Mn-SOD.
[0058] The sequence obtained above was digested with NdeI / HindIII and inserted into the corresponding site of the pET28a expression vector (purchased from Invitrogen), and sequenced to identify the correctly inserted recombinant expression vector. The recombinant expression vector was transformed into Escherichia coli BL21(DE3) by conventional methods to obtain transformants.
[0059] Pick the monoclonal colony on the transformation plate, inoculate it into 30mL LB culture medium (containing 100μg / mL Amp), place it on a shaker at 37°C for overn...
Embodiment 2
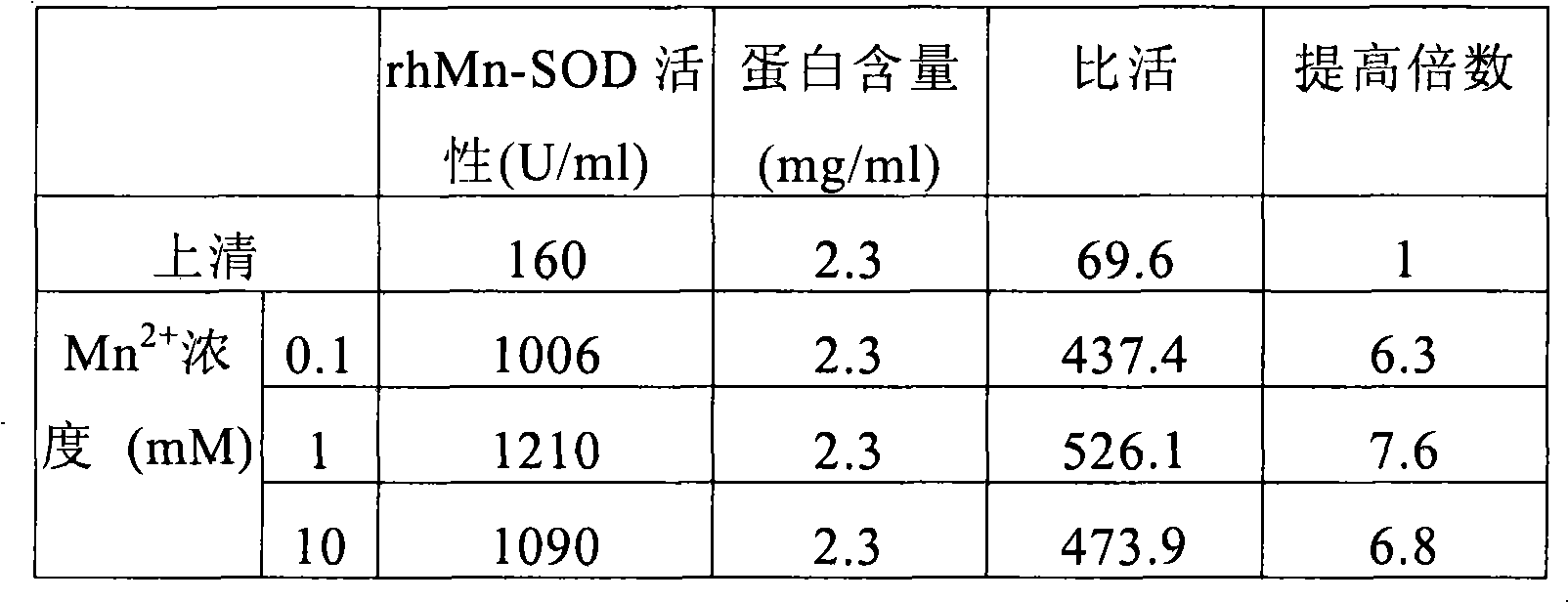
[0060] Embodiment 2, adding different concentrations of Mn in the supernatant 2+ Effect on Mn-SOD activation
[0061] The cell culture obtained above was ultrasonically disrupted, and the supernatant was collected after centrifugation, which contained soluble recombinant human Mn-SOD and other miscellaneous proteins.
[0062] Add different concentrations of Mn to the supernatant containing soluble recombinant human Mn-SOD (rhMn-SOD) 2+ , observe different concentrations of Mn 2+ Effect on Mn-SOD activation. The results are shown in Table 1.
[0063] The determination method of rhMn-SOD activity adopts the trace pyrogallol method. Enzyme activity is defined as follows: at 25° C., the amount of enzyme that inhibits the autoxidation rate of pyrogallol to 50% per minute in 1 ml of reaction solution is an activity unit.
[0064] The determination of rhMn-SOD specific activity is defined as: the number of enzyme activity units contained in each mg protein. The unit is U / mg.
...
Embodiment 3
[0068] Embodiment 3, thermal denaturation method removes impurity protein in supernatant
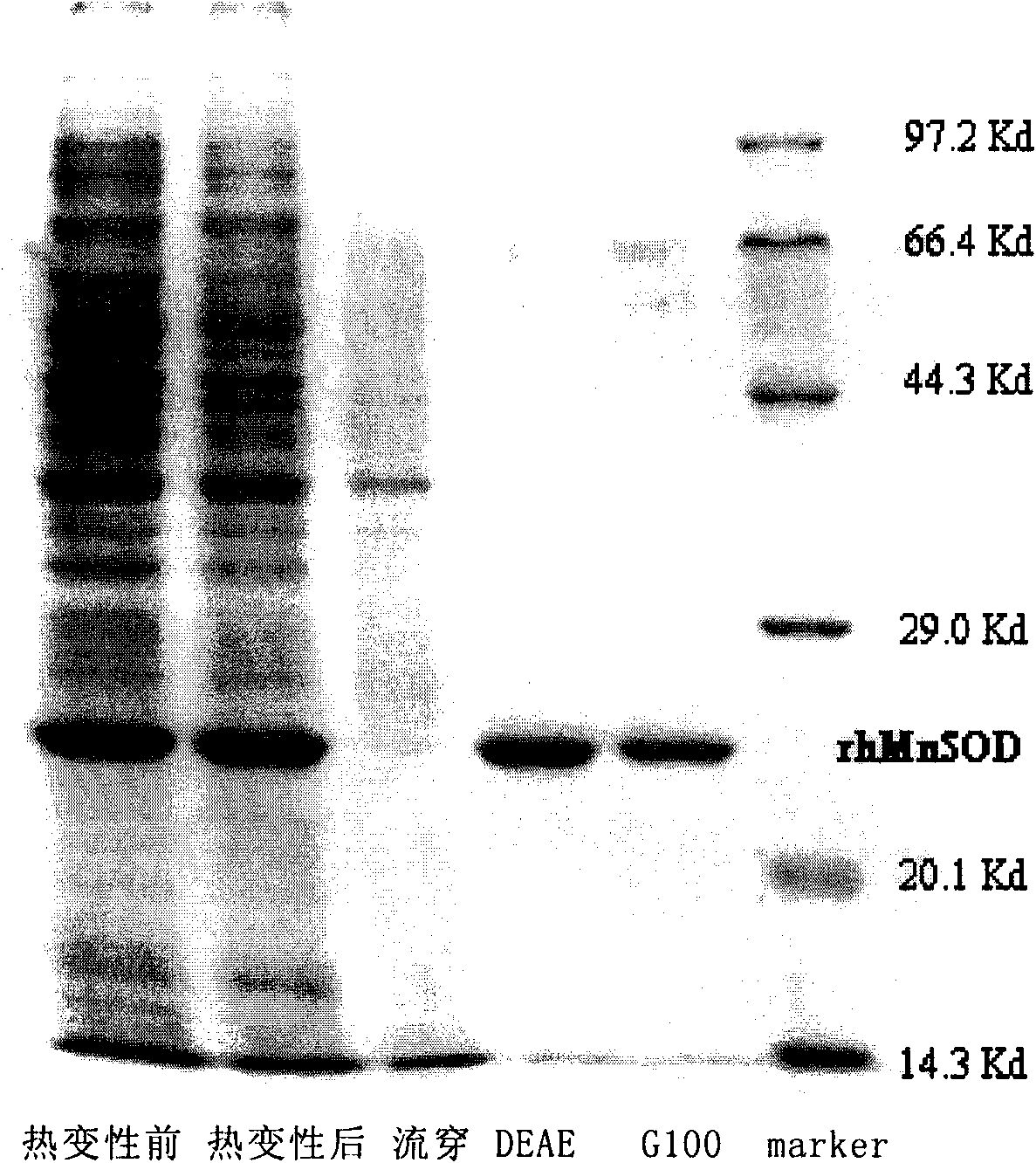
[0069] The cell culture obtained above was ultrasonically disrupted, and the supernatant was collected after centrifugation to obtain a crude enzyme solution.
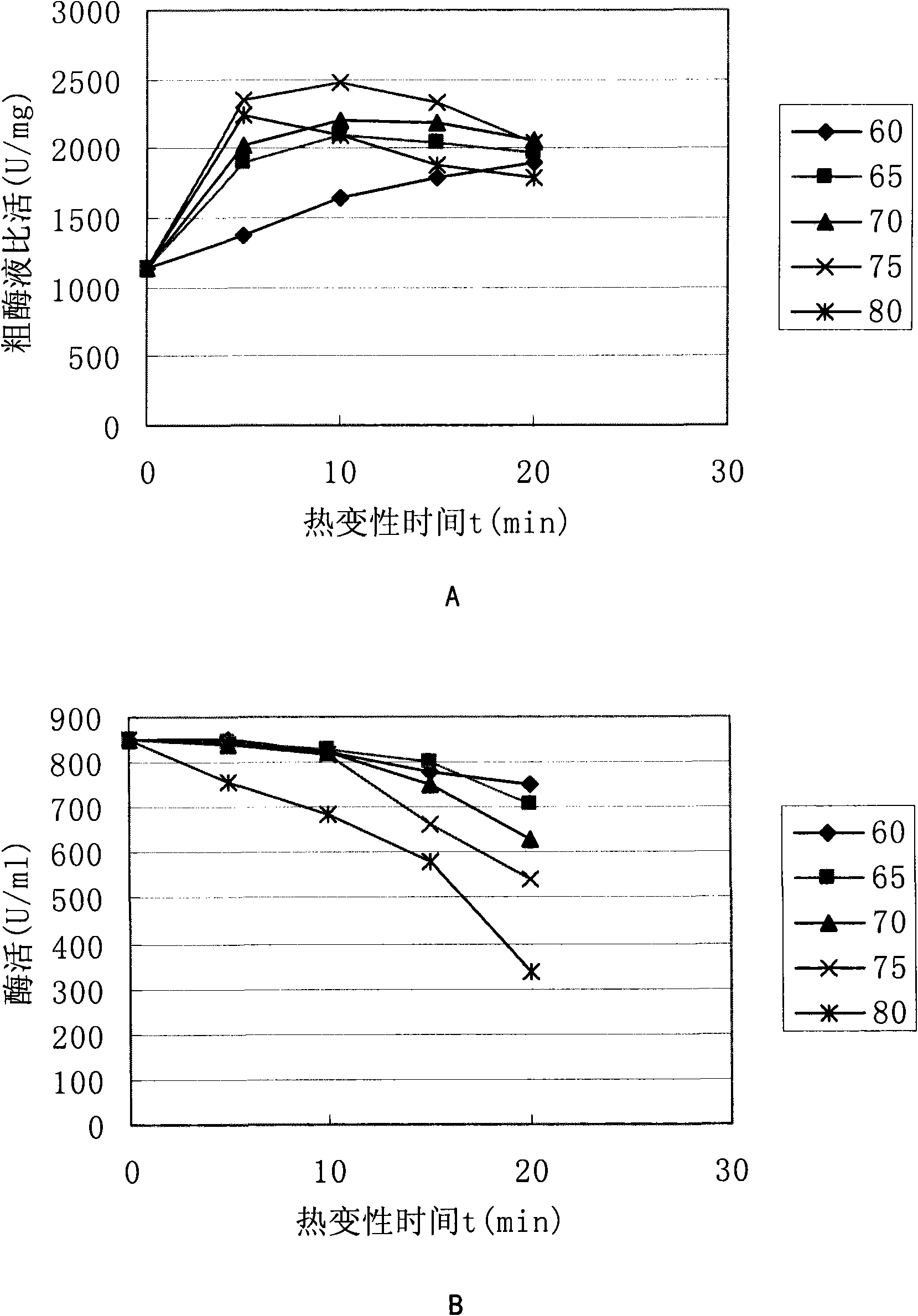
[0070] The crude enzyme solution obtained after ultrasonically crushing the bacteria was subjected to heat denaturation for 5, 10, 15, and 20 minutes in a water bath at 60, 65, 70, 75, and 80°C, respectively, and immediately immersed in water (water temperature 0°C) to cool after heat denaturation, and centrifuged After 10 min, take the supernatant enzyme solution and measure the specific activity. The obtained specific activity assay results are shown in figure 1 a.
[0071] It can be seen that the thermal denaturation effect at 75°C for 10 minutes is the best, and the specific activity in the supernatant is increased by 2.5 times, and the total enzyme activity is not significantly reduced at this time, see figure 1 b.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com