Efficacy test method of infectious bronchitis vaccines and application thereof
A technology for bronchitis and chicken infectivity, applied in the biological field, can solve the problems of poor controllability, error in test results, long time, etc., and achieve the effect of small batch difference, small error and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Efficacy test method of chicken infectious bronchitis inactivated vaccine
[0055] 1. Vaccination
[0056] 10 3-week-old SPF chickens were inoculated with chicken infectious bronchitis live vaccine (H 120 strain) 1 part (10 3.5 EID 50 ), after 21 days, blood was collected respectively, and serum was separated, and 1 feather portion (0.5ml) was boosted with chicken infectious bronchitis inactivated vaccine (intramuscular injection) respectively, and blood was collected respectively after 28 days, and serum was separated. The HI antibody titers were measured in the two sera respectively.
[0057] 2. Chicken infectious bronchitis hemagglutination inhibition (HI) test
[0058] 1. Determination of hemagglutination value of chicken infectious bronchitis HI antigen
[0059] (1) Take 25 μl of PBS with a concentration of 0.01mol / L and a pH of 7.2, and add it to each well of the V-shaped microreaction plate;
[0060] (2) Take 25 μl chicken infectious bronchitis hemagglutina...
Embodiment 2
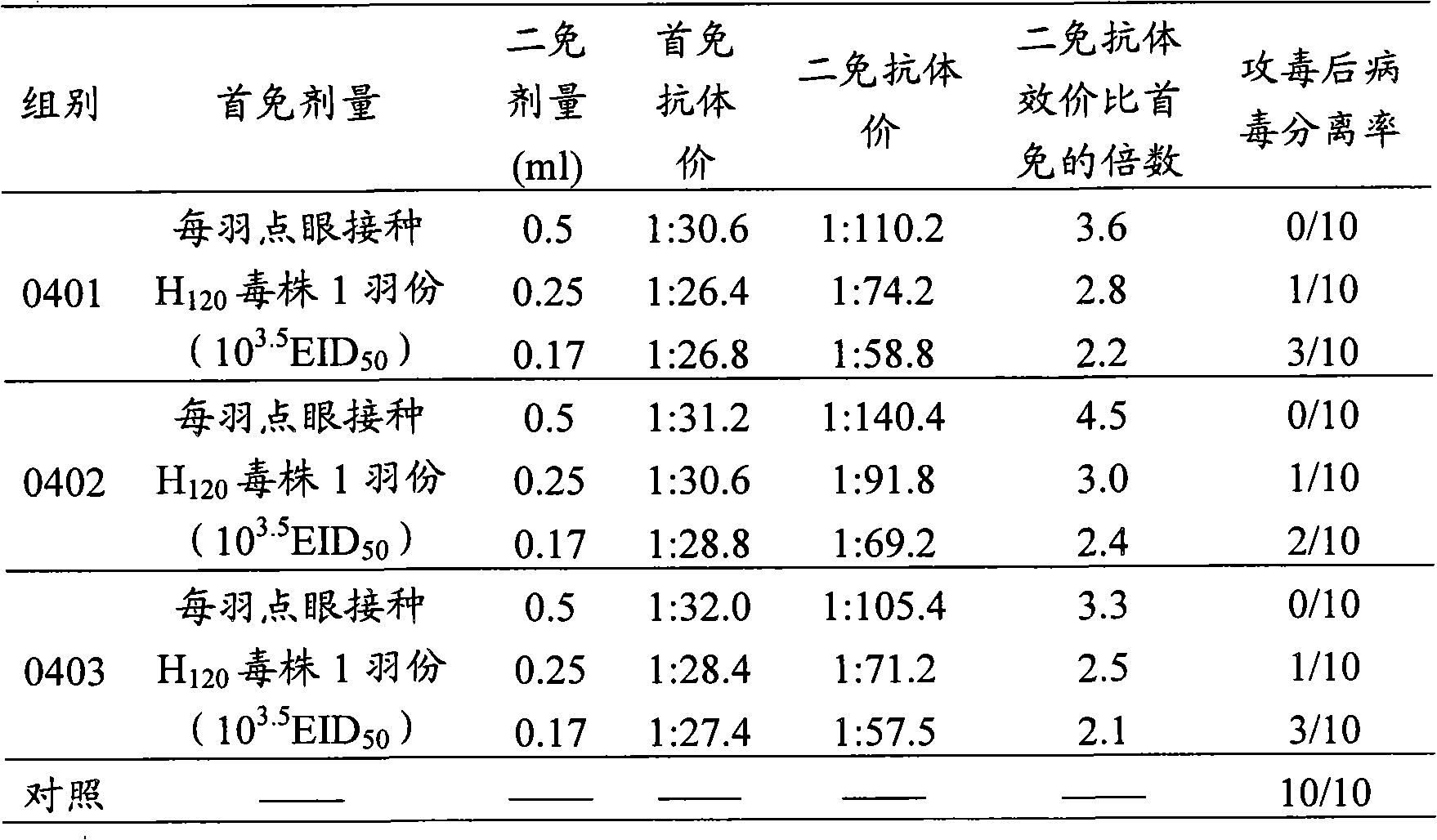
[0081] Parallel relationship between HI antibody titer and challenge protection rate in experimental animals immunized with chicken infectious bronchitis inactivated vaccine
[0082] With 3 batches of vaccines, every batch of 30 21-day-old SPF chickens is divided into 3 groups, 10 in every group, and each eye is inoculated with infectious bronchitis live vaccine (H 120 strain) 1 part (10 3.5 EID 50 ), raised and observed in a positive pressure isolator (10 non-immunized control groups were set up at the same time), blood was collected and separated serum was tested for HI antibody on 21 days after immunization, and each chicken was injected intramuscularly with chicken infectious bronchitis inactivated vaccine to strengthen immunization at the same time. The first group is 1 feather portion (0.5ml), the second group is 1 / 2 feather portion (0.25ml), and the third group is 1 / 3 feather portion (0.17ml). After 28 days of booster immunization, blood was collected and serum was se...
Embodiment 3
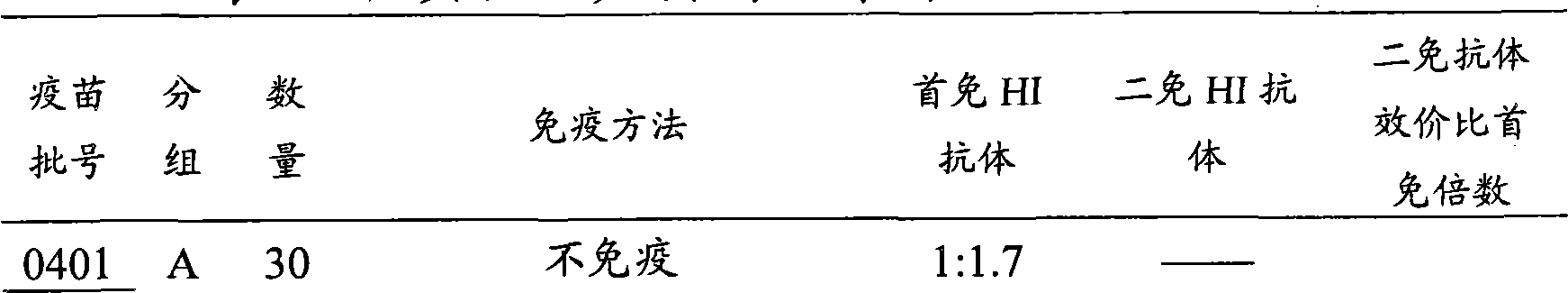
[0090] The Parallel Relationship Between HI Antibody Titer and Egg Production Efficiency of Animals Immunized with Inactivated Infectious Bronchitis Vaccine
[0091] With 3 batches of vaccines, each batch was divided into 5 groups, and each group used 30 143-day-old non-immunized chickens, and the grouping experiment was carried out according to the following method.
[0092] Group A: no immunization;
[0093] Group B: 0.8ml of inactivated vaccine was subcutaneously inoculated into each chicken at the age of 164 days;
[0094] Group C: each intranasal inoculation of chicken infectious bronchitis live vaccine (H 120 ) 1 feather portion (10 3.8 EID 50 ), after 21 days of age, blood was collected, serum was separated, HI antibody was measured to calculate the geometric mean titer of HI antibody, and then 0.8ml of inactivated vaccine was subcutaneously injected at 164 days of age;
[0095] Group D: at the age of 143 days, the chicken infectious bronchitis live vaccine (H 120 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com