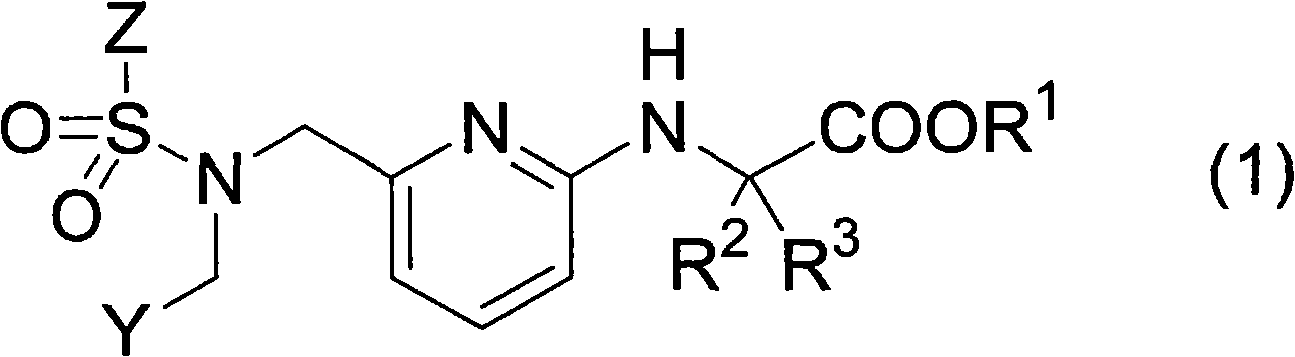
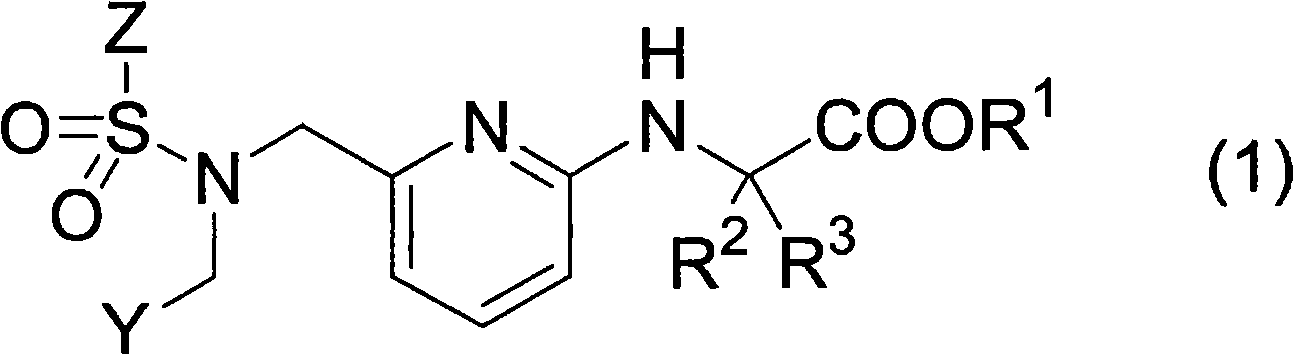
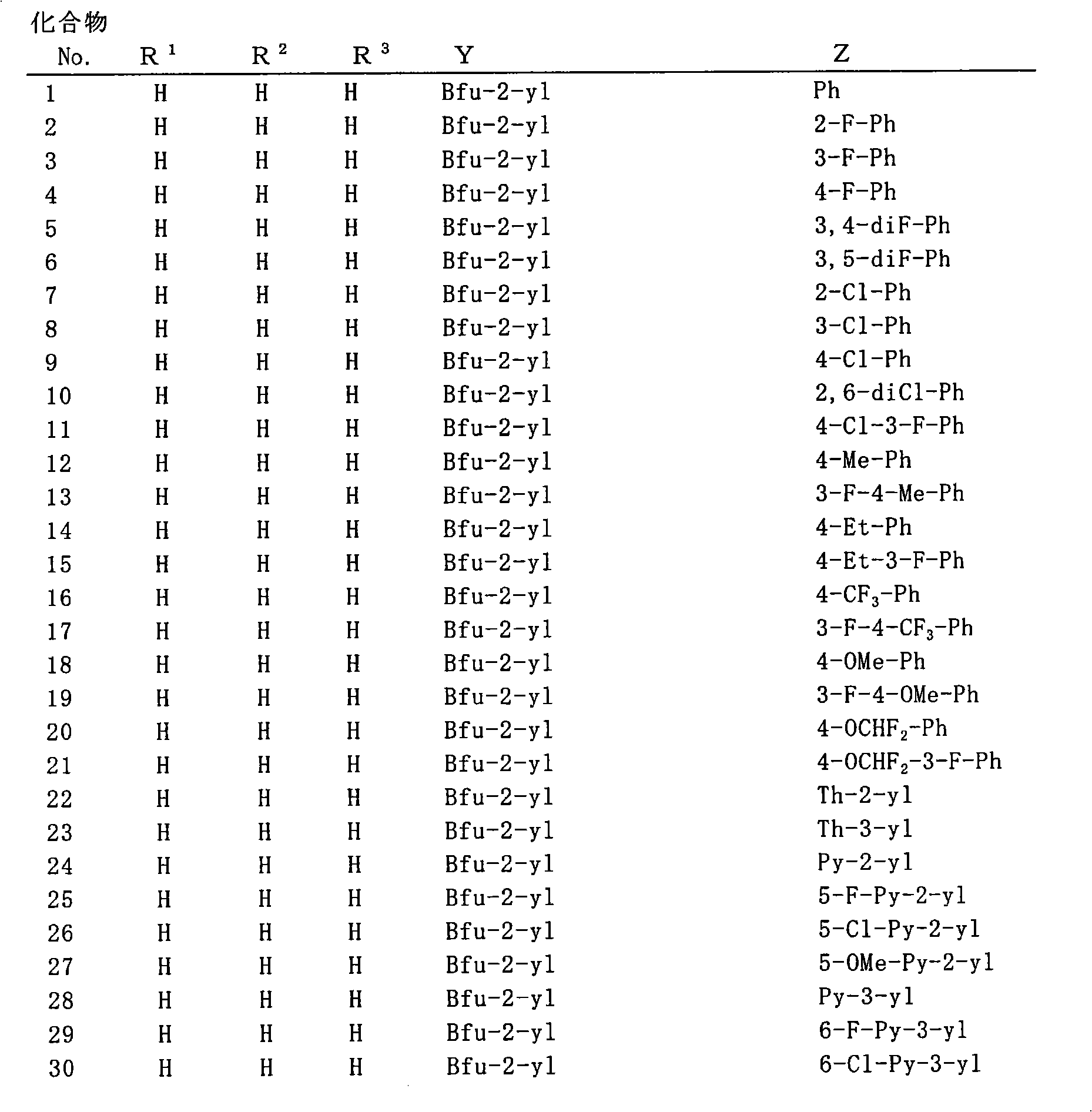
Pyridylaminoacetic acid compound
A kind of technology of pyridylamino, pyridylamino, applied in the field of pyridylamino acetic acid compounds, can solve the problems such as no specific disclosure of sulfonamide compounds, and achieve the effect of excellent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0747] The present invention will be described in more detail below with examples, reference examples, and test examples, but the scope of the present invention is not limited thereto.
[0748] [Example 1]
[0749] {6-[(6-phenylpyridazin-3-ylmethyl)(pyridin-3-ylsulfonyl)aminomethyl] Pyridin-2-ylamino}acetic acid hydrochloride (Exemplary Compound No. 1397)
[0750] 1-(a) ({5-bromo-6-[(6-phenylpyridazin-3-ylmethyl)(pyridin-3-ylsulfonate Acyl)aminomethyl]pyridin-2-yl}tert-butoxycarbonylamino)tert-butyl acetate
[0751] 1.75 ml of N,N-dimethylformamide of 114 mg (0.349 mmol) of N-(6-phenylpyridazin-3-ylmethyl)pyridin-3-ylsulfonamide obtained in Reference Example 2-(d) In the solution, add [(5-bromo-6-bromomethylpyridin-2-yl) tert-butoxycarbonylamino] tert-butyl acetate 233mg (containing 0.35mmol in terms of pure components) obtained in Reference Example 1-(c). ) and potassium carbonate 98.0mg (0.709mmol), stirred at room temperature for 20 hours. After the reaction, 5.3 ...
Embodiment 2
[0764] (6-{(pyridin-3-ylsulfonyl)[4-(thiazol-2-yl)benzyl]aminomethyl} Pyridin-2-ylamino)acetic acid (Exemplary Compound No. 985)
[0765] 2-(a) [tert-butoxycarbonyl(6-{(pyridin-3-ylsulfonyl)[4-(thiazole-2 -yl)benzyl]aminomethyl}pyridin-2-yl)amino]tert-butyl acetate
[0766] In the tetrahydrofuran 20ml solution of the N-[4-(thiazol-2-yl) benzyl]pyridin-3-yl sulfonamide 686mg (2.07mmol) obtained in the reference example 4-(e), add the reference example 3-(b ) obtained in [tert-butoxycarbonyl (6-hydroxymethylpyridin-2-yl) amino] tert-butyl acetate 743mg (2.20mmol), tri-n-butylphosphine 980μl (3.92mmol) and N, N, N 562 mg (3.26 mmol) of ', N'-tetramethylazodicarbonamide was stirred at room temperature for 11 hours. After the reaction was completed, saturated aqueous sodium chloride solution was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer after liquid separation was washed with a saturated aqueous sodium chloride solution, dr...
Embodiment 3
[0777] (6-{(pyridin-2-ylsulfonyl)[4-(thiazol-2-yl)benzyl]aminomethyl} Pyridin-2-ylamino)acetic acid (Exemplary Compound No. 977)
[0778] 3-(a) [tert-butoxycarbonyl(6-{(pyridin-2-ylsulfonyl)[4-(thiazole-2 -yl)benzyl]aminomethyl}pyridin-2-yl)amino]tert-butyl acetate
[0779] In addition to using 279 mg (0.824 mmol) of [tert-butoxycarbonyl (6-hydroxymethylpyridin-2-yl) amino] tert-butyl acetate obtained in Reference Example 3-(b), and using tert-butyl acetate obtained in Reference Example 5 N-[4-(thiazol-2-yl)benzyl]pyridin-2-ylsulfonamide 275mg (0.830mmol) instead of N-[4-(thiazol-2-yl)benzyl]pyridin-3-ylsulfonamide, The reaction and post-treatment were carried out according to Example 2-(a), to obtain 496 mg of the title compound in the form of white foam. (yield is 92%)
[0780] Mass spectrum (FAB, m / z): 652 (M + +1).
[0781] 1 H-NMR spectrum (CDCl 3 , δppm): 8.60 (ddd, J = 4.7, 1.7, 0.9Hz, 1H), 7.85 (d, J = 3.1Hz, 1H), 7.85-7.81 (m, 3H), 7.77 (ddd, J = 7.7, 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com