Preparation method and applications of anti-latent membrane protein LMP2A monoclonal antibody
A monoclonal antibody, latent membrane protein technology, applied in the field of medical molecular biology, can solve the problems of poor curative effect and poor prognosis of nasopharyngeal carcinoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Design of primers for amplifying the full-length sequence of LMP2A and the amplification of the full-length sequence of LMP2A
[0034] According to the principle of primer design, it is designed using the software Primer5.0, and the full-length sequence of LMP2A can be amplified after experimental verification. The detailed primer sequence is as follows:
[0035] up-LMP2A-BglII:
[0036] 5'gaagatctatggggtccctagaaatggt3';
[0037] up-flag-LMP2A-BglII:
[0038] 5'gaagatctaccatggactacaaggacgacgatgacaaggggtccctagaaatggtgc3';down-flag-LMP2A-EcoR I:
[0039] 5'cggaattcttacttgtcatcgtcgtccttgtagtctacagtgttgcgatatggg3'
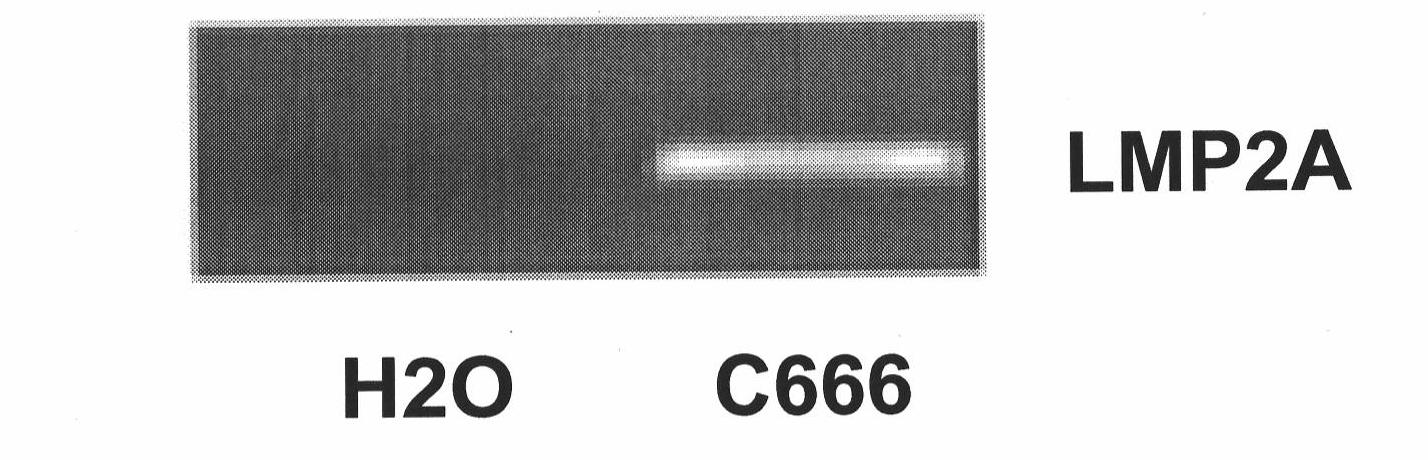
[0040] The specific experimental steps, conditions and electropherograms for amplifying the full-length sequence of LMP2A are as follows:
[0041] RT (reverse transcription)
[0042] (1) Take 2 μg of RNA from C666 cells, and add samples one by one according to the following system (volume 15 μl): RNA 2 μg, random primer 1 μl water (μl) to balance...
Embodiment 2
[0063] The anti-LMP2A monoclonal antibody prepared in Example 2 detects the expression of LMP2A in nasopharyngeal carcinoma tissue
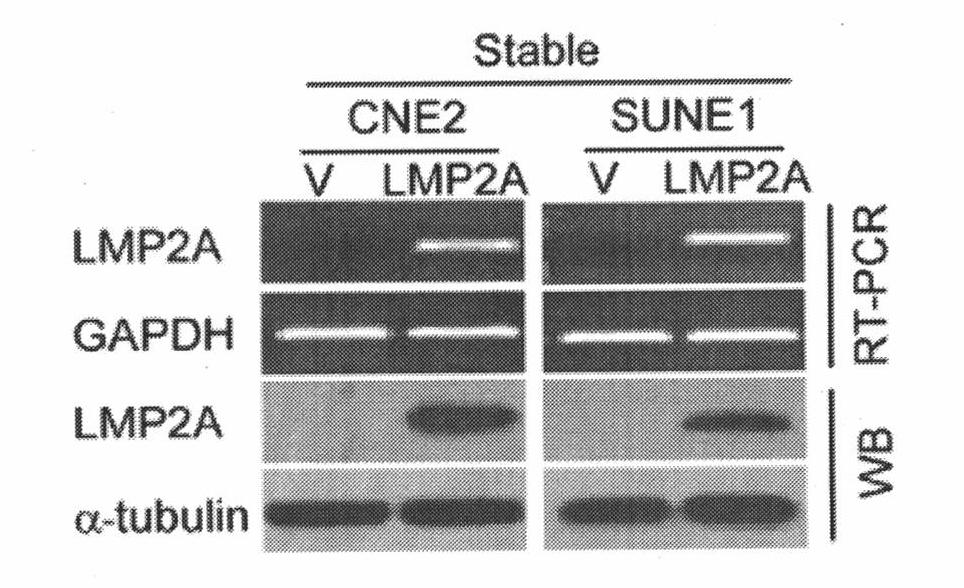
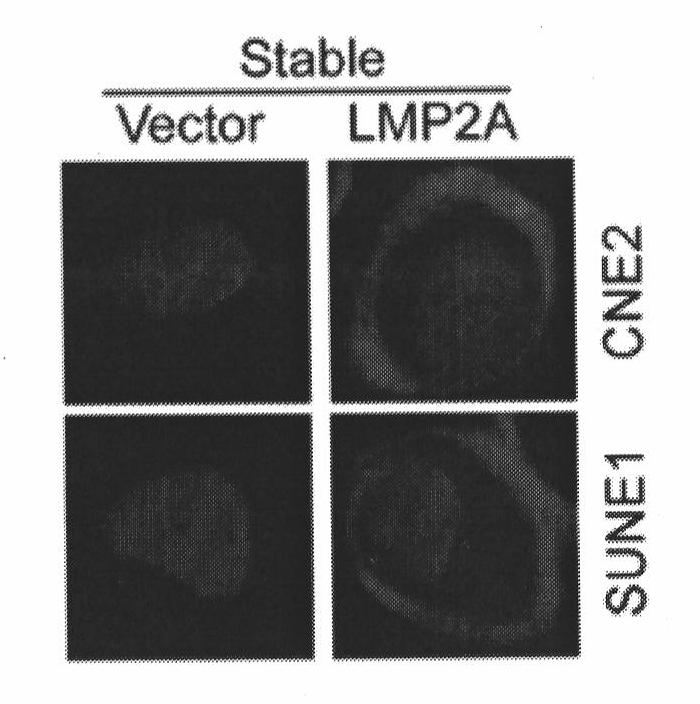
[0064] 1. Establishment of stable cell lines exogenously expressing LMP2A and identification of LMP2A antibodies
[0065] Nasopharyngeal carcinoma cells CNE2 and SUNE1 in the logarithmic growth phase were taken, and the two cell lines were infected with Pbabe and Pbabe-LMP2A viruses at the same time, and were screened with a medium containing 1ug / ml puromycin for 3 days, and passed down. CNE2-Vector, CNE2-LMP2A, SUNE1-Vector, SUNE1-LMP2A cells were lysed with protein lysate, and protein quantification was performed by BCA method. Denature at 98°C for 10 minutes, load 20ug of the sample for western blotting detection; at the same time, use Trizol to lyse the cells, extract RNA, and perform RT-PCR detection. figure 2 It is the establishment of a stable cell line exogenously expressing LMP2A, and the expression of its RNA and protein. From this we...
Embodiment 3
[0073] Example 3 LMP2A is used as a target, and monoclonal antibodies are used for specific neutralization to treat nasopharyngeal carcinoma
[0074] 1. In vitro detection of monoclonal antibody on the tumorigenicity of LMP2A-expressing cells and its ability to reverse EMT.
[0075] 1.1 Incubate with monoclonal antibody and endogenous and exogenous expression LMP2A cells, and use MTT method to detect cell proliferation
[0076] The cells C666 and CNE2-LMP2A were digested and cultured in a 96-well plate. Antibodies were added to the cells at a certain concentration, and a blank control group was set. The MTT method was used to monitor for 7 days to analyze the proliferation of the cells. Such as Figure 5 As shown, monoclonal antibodies can inhibit cell proliferation. (P<0.05)
[0077] 1.2 Using plate cloning and soft agar colony formation experiments to observe the transformation ability of cells treated with antibodies
[0078] After incubating the antibody with cells C66...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com