Method for measuring lead, arsenic, nickel and cobalt in ferric oxide powder
A technology of iron oxide powder and constant volume, which is applied in the field of arsenic, nickel and cobalt determination of lead in iron oxide powder, can solve the problems of unprovided, labor-intensive, cumbersome operation process, etc., and achieve accurate and concise operation. Fast analysis speed and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
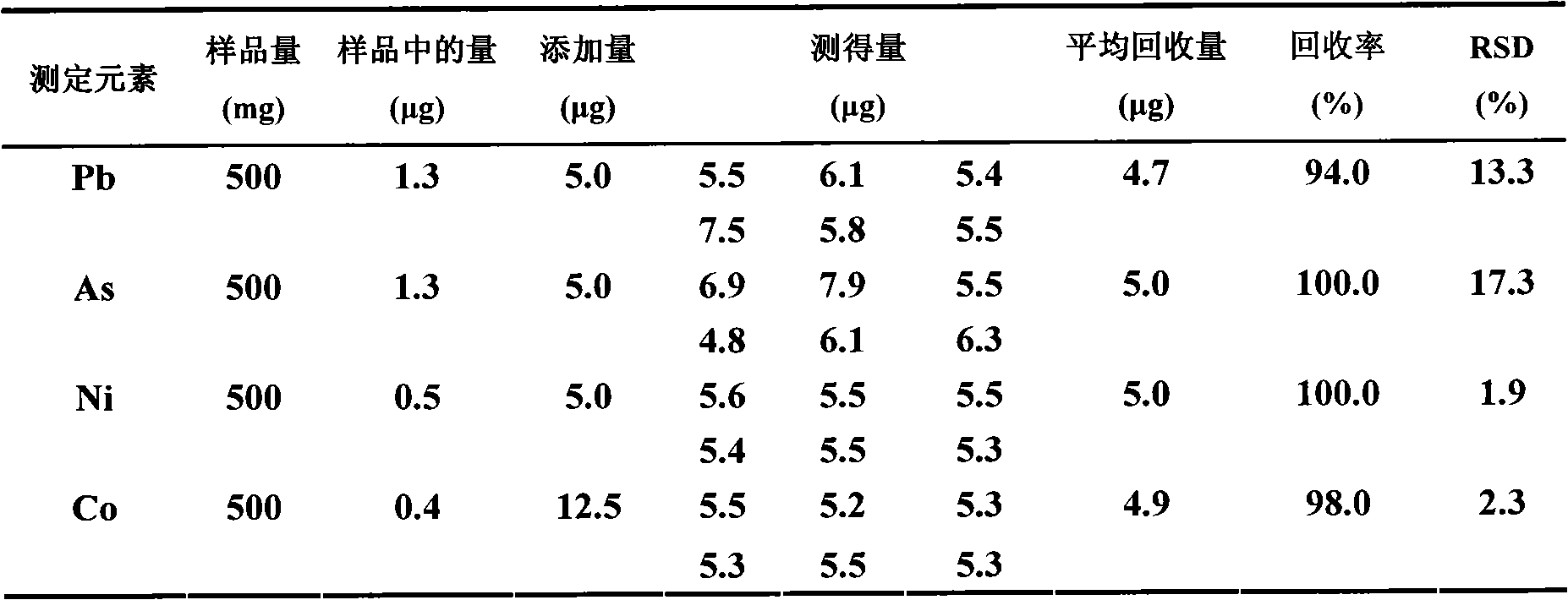
Embodiment 1
[0023] A) Sampling and dissolving, weigh 0.50g of iron oxide powder sample, and place the weighed 0.50g iron oxide powder sample in the first container served by a beaker with a volume of 100mL, and add concentration 25mL of 8mol / L hydrochloric acid, and heated to boiling, then 1mL of hydrogen peroxide was added dropwise to completely dissolve the iron oxide powder, and after cooling, the volume of the dissolved sample was controlled to be 25mL (due to the addition of 25mL of hydrochloric acid and 1mL of hydrogen peroxide when heated loss);
[0024] B) Through the column, the dissolved sample obtained from step A) is introduced into the extraction resin column with a particle size of 60-75 mesh extraction resin under the condition that the flow rate is controlled at 0.5mL / min, and the outflow from the extraction resin column is discarded. 5mL of the effluent of the first part of the first part, and the first part of the effluent was introduced into the container for discarding...
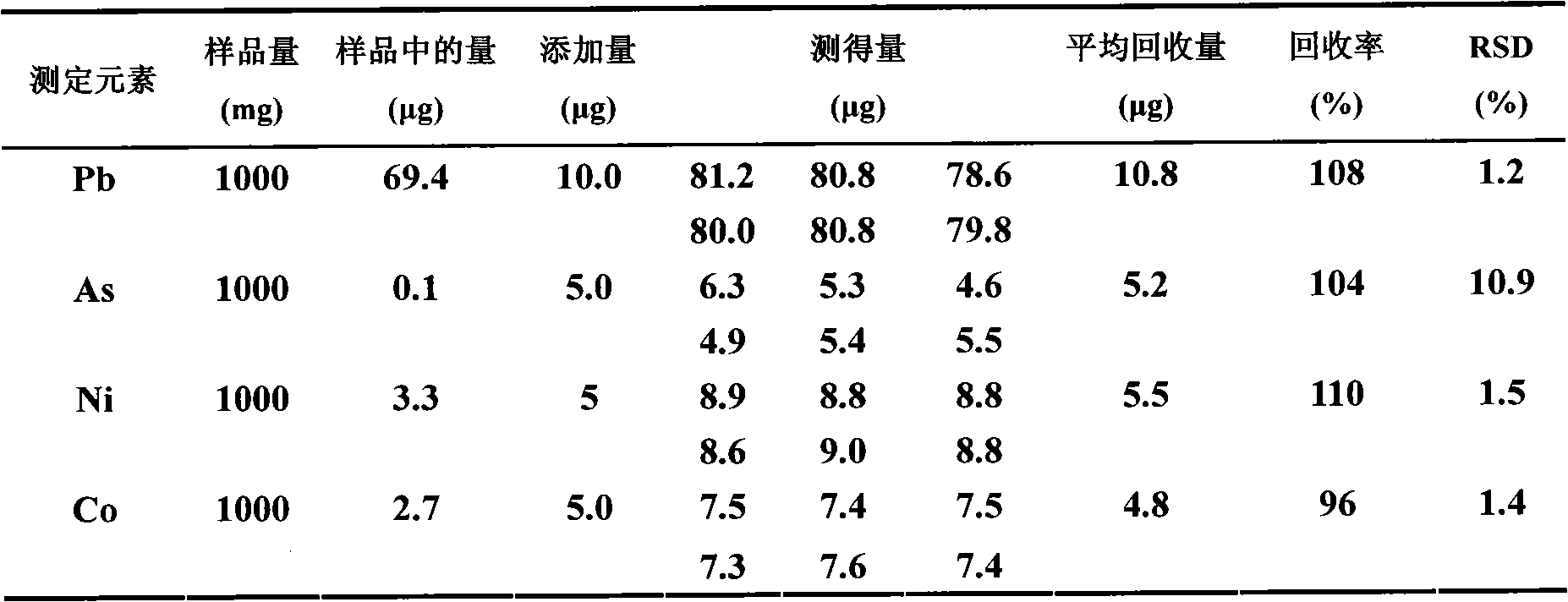
Embodiment 2
[0030] A) Sampling and sample dissolution, weigh 1.00g of iron oxide powder sample, and place the weighed 1.00g iron oxide powder sample in the first container served by a beaker with a volume of 100mL, and add concentration 60mL of 7mol / L hydrochloric acid, and heated to boiling, then dropwise added 3mL of hydrogen peroxide to completely dissolve the iron oxide powder, after cooling, the volume of the dissolved sample was controlled to be 60mL (due to the addition of 60mL of hydrochloric acid and 3mL of hydrogen peroxide when heated loss);
[0031] B) Through the column, the dissolved sample obtained from step A) is introduced into the extraction resin column with a particle size of 60-75 mesh extraction resin under the condition that the flow rate is controlled at 1.0mL / min, and the outflow from the extraction resin column is discarded. 21mL of the effluent of the first part of the first part, and the first part of the effluent was introduced into the container for discardin...
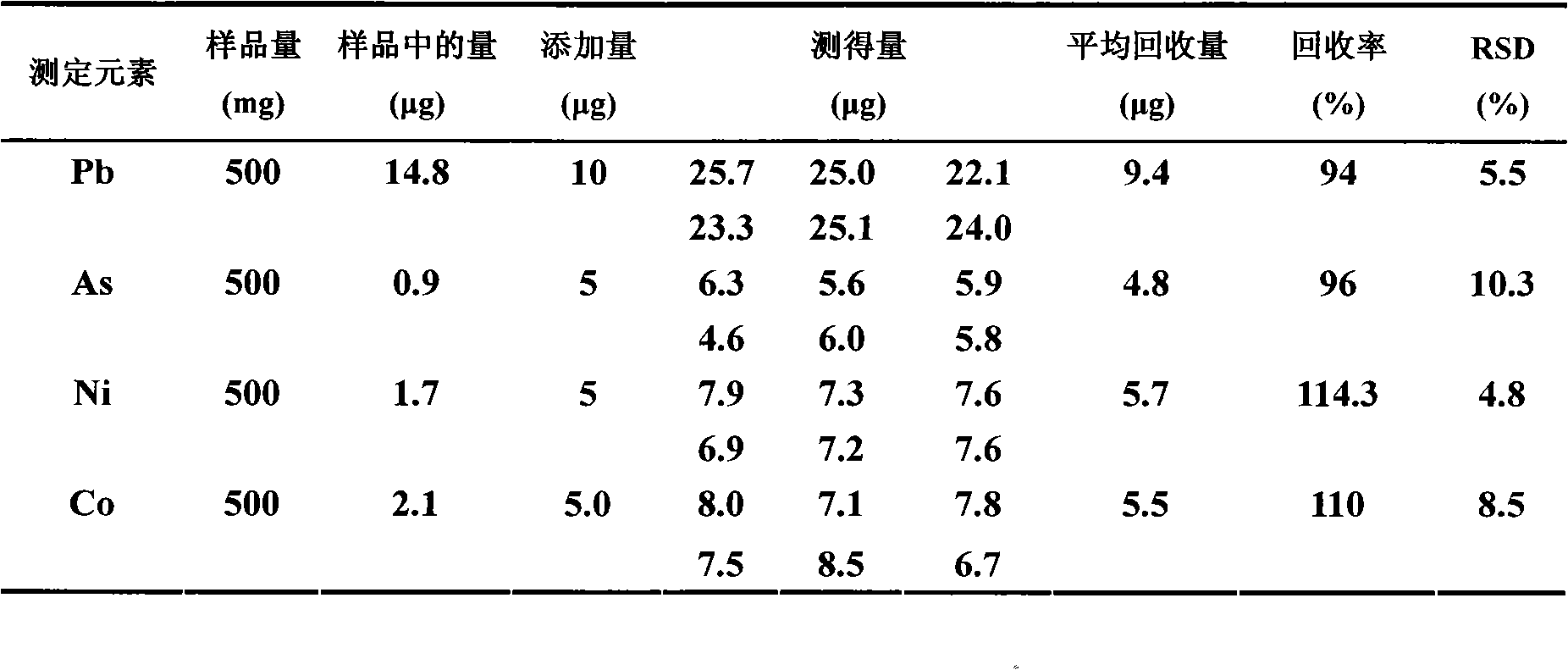
Embodiment 3
[0037] A) Sampling and dissolving, weigh 0.50g of iron oxide powder sample, and place the weighed 0.50g iron oxide powder sample in the first container served by a beaker with a volume of 100mL, and add concentration 40mL of 6mol / L hydrochloric acid, and heated to boiling, then dropwise added 1mL of hydrogen peroxide to completely dissolve the iron oxide powder, after cooling, control the volume of the dissolved sample to 40mL (due to the addition of 40mL of hydrochloric acid and 1mL of hydrogen peroxide when heated loss);
[0038] B) Through the column, the dissolved sample obtained from step A) is introduced into the extraction resin column with a particle size of 60-75 mesh extraction resin under the condition that the flow rate is controlled at 0.7mL / min, and the outflow from the extraction resin column is discarded. 10mL of the first part of the effluent, and the first part of the effluent was introduced into the container for disposal, and the remaining effluent was coll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com