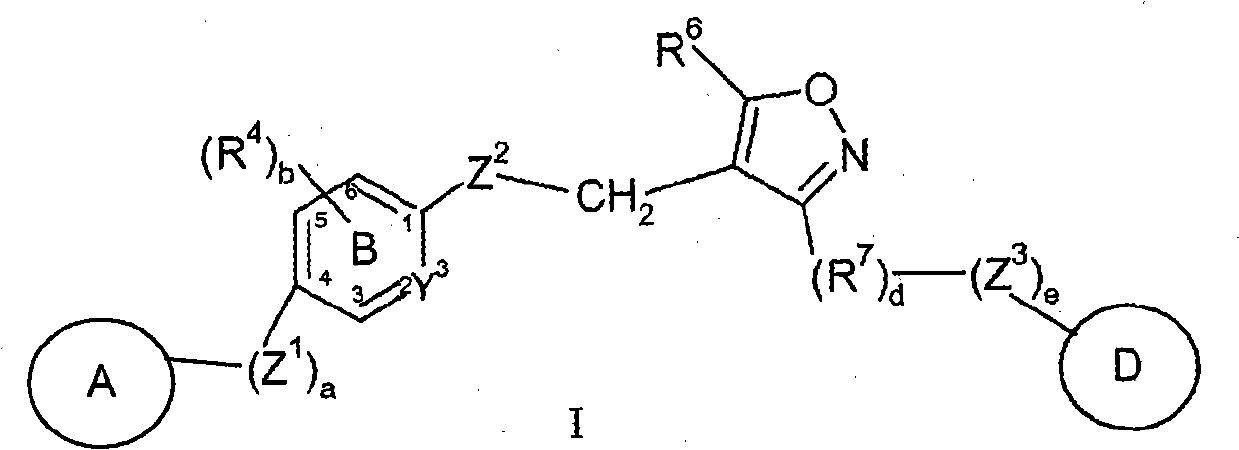
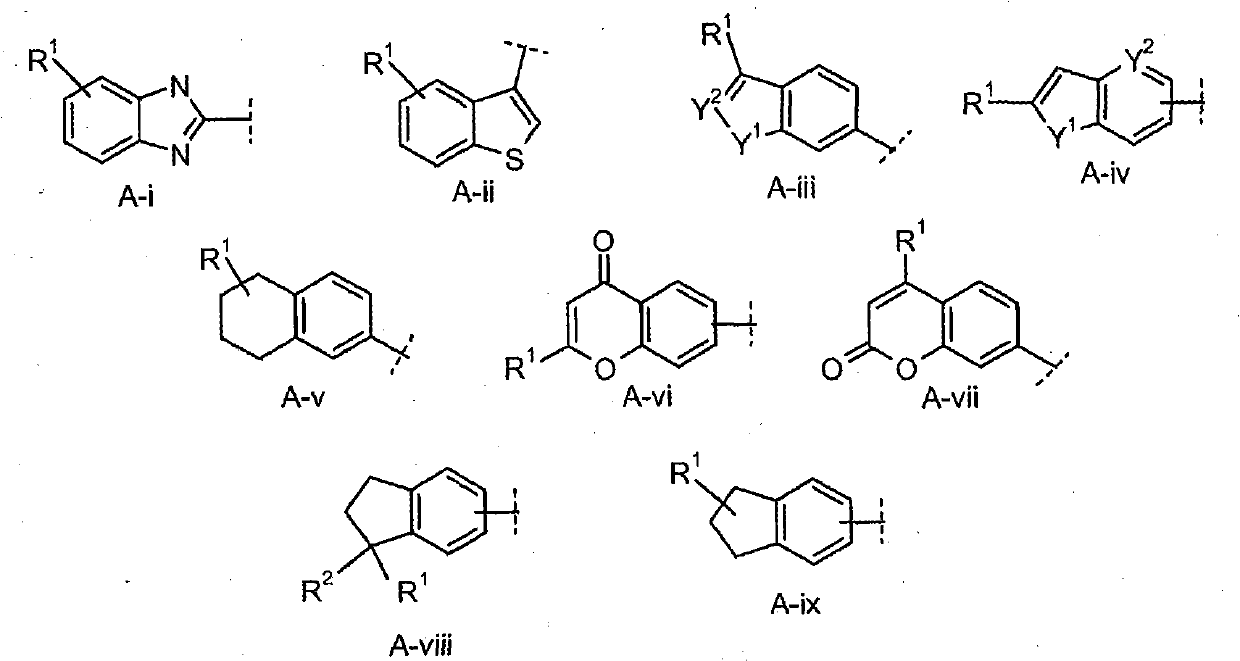
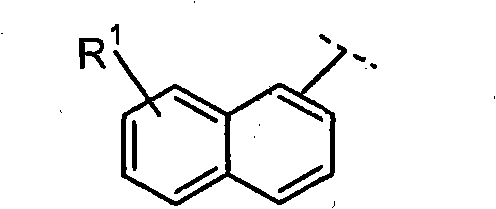
Farnesoid x receptor agonists
A technology for subjects and mammals, applied in the field of farnesoid X receptors in the case of a fatty diet, can solve the problem that the function of ileal bile acid binding protein needs to be studied and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0544] Example 1: 5-[4-({[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methyl}oxy)benzene base]-1H-indole-2-carboxylic acid
[0545]
[0546] 1a) 1-(1,1-dimethylethyl) 5-{[(trifluoromethyl)sulfonyl]oxy}-1H-indole-1,2-dicarboxylate · 2-ethyl ester
[0547]
[0548] Di-tert-butyl dicarbonate (1.78 g, 8.13 mmol) and N,N-dimethylaminopyridine (83 mg, 0.68 mmol) were added to 5-[(phenylmethyl) in tetrahydrofuran (20 mL) at room temperature Oxy]-1H-indole-2-carboxylic acid ethyl ester (2.0 g, 6.77 mmol). After stirring for 1.5 hours, ethyl acetate was added, and the mixture was washed with water and brine, then dried over sodium sulfate. The solution was concentrated, and the residue was taken up in methanol (40 mL) and chloroform (40 mL). Palladium on carbon (10%, 100 mg) was added and the mixture was shaken under hydrogen (40 psi) in a Parr apparatus for 1 h at room temperature. Pass the solution through Pad filter, then concentrate. Dichloromethane (20 mL) w...
Embodiment 2
[0558] Example 2: 6-[4-({[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methyl}oxy)benzene base]-2,3-dihydro-1H-indene-1-carboxylic acid
[0559]
[0560] 2a) Methyl 6-hydroxy-2,3-dihydro-1H-indene-1-carboxylate
[0561]
[0562] A solution of 1,3-dithiane (2.37 g, 19.7 mmol) in tetrahydrofuran (30 mL) was cooled to -15°C and n-butyllithium (2.5M in hexane, 7.40 mL, 18.5 mmol) was added. After stirring at -15°C for 1 hour, 6-(methyloxy)-2,3-dihydro-1H-inden-1-one (2.0 g, 12.3 mmol) in tetrahydrofuran (75 mL) was added dropwise, and then The mixture was warmed to room temperature over 3 hours. Ethyl acetate was added, the mixture was washed with water and brine, then dried over sodium sulfate and concentrated. The residue was taken up in toluene (75 mL) and p-toluenesulfonic acid (350 mg, 1.85 mmol) was added. The mixture was heated to reflux in a Dean Stark apparatus for 1.5 hours, then passed Plug filtered and concentrated. The residue was purified by sil...
Embodiment 3
[0573] Example 3: 6-[4-({[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methyl}oxy)benzene base]-1H-indole-3-carboxylic acid
[0574]
[0575] 3a) 1-(1,1-dimethylethyl) 6-{[(trifluoromethyl)sulfonyl]oxy}-1H-indole-1,3-dicarboxylate · 3-ethyl ester
[0576]
[0577] Ethyl 6-[(phenylmethyl)oxy]-1H-indole-3-carboxylate in chloroform (10 mL) and methanol (5 mL) [according to Bioorg.Med.Chem, 9 (8) 2119 (2001 ) preparation] (350 mg, 1.19 mmol) and palladium / carbon (10%, 100 mg) mixture was vigorously stirred under hydrogen (1 atm) for 1.5 h. Pass the mixture through Pad filter, then concentrate. The residue was taken up in dichloromethane (8 mL), cooled to 0 °C, triethylamine (250 μL, 1.78 mmol) was added followed by trifluoromethanesulfonic anhydride (240 μL, 1.42 mmol). After stirring at room temperature for 16 hours, the mixture was concentrated and the residue was taken up in ethyl acetate. The organics were washed with water and brine, then concentrated. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com