Influenza virus hemagglutinin fusion peptide-containing targeted recombinant molecule gene, protein encoded thereby and application thereof
An influenza virus and hemagglutinin technology, applied in the field of gene therapy, can solve the problem of reducing the efficacy of antibody fusion proteins, and achieve the effects of small immunogenicity, direct killing effect, and high-efficiency apoptosis-promoting biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Construction of HBsAg targeting pro-apoptotic molecular genes
[0024] (1) Construction of pET32a-scFv-Fu-HAfp-tBid plasmid (Fu is the furin protease recognition site, the same below)
[0025] With the plasmid pET32a-HBV-scFv preserved in the inventor's laboratory as a template (Wen WH, Qin WJ, Gao H, Zhao J, Jia LT, Liao QH, Meng YL, Jin BQ, Yao LB, Chen SY, YangAG.An hepatitis B virus surface antigen specific single chain of variable fragment derived from a natural immune antigen binding fragment phage display library is specifically internalized by HepG2.2.15 cells.J Viral Hepat.2007 Jul; 14(7):512-9.), with HBV -scFv-p5 and HAfp-1st-p3 are used as upstream and downstream primers for the first round of amplification, and the obtained PCR product is then used for the second round of amplification with HBV-scFv-p5 and HAfp-2nd-p3 as upstream and downstream primers , to obtain scFv-HAfp fragments. The scFv-HAfp fragment was double-digested with NcoI and EcoRI, and ...
Embodiment 2
[0044] 2. Construction of HER2 targeting pro-apoptotic molecular genes
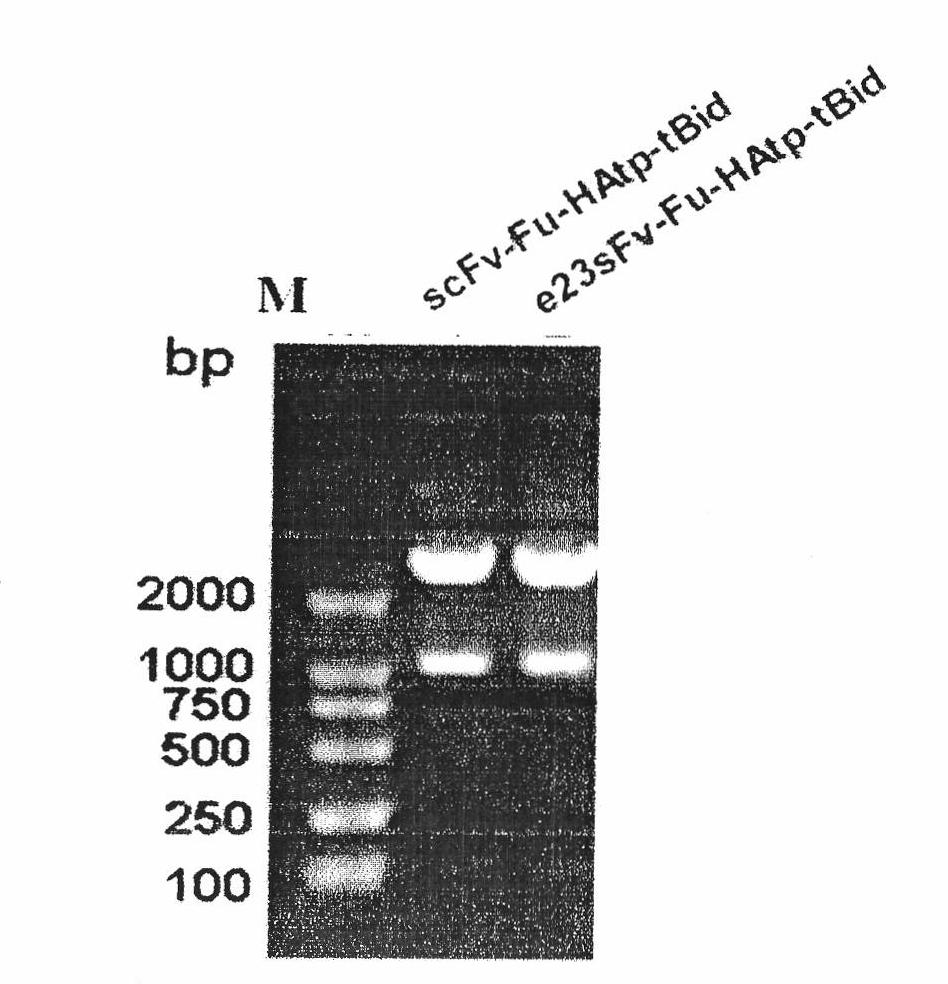
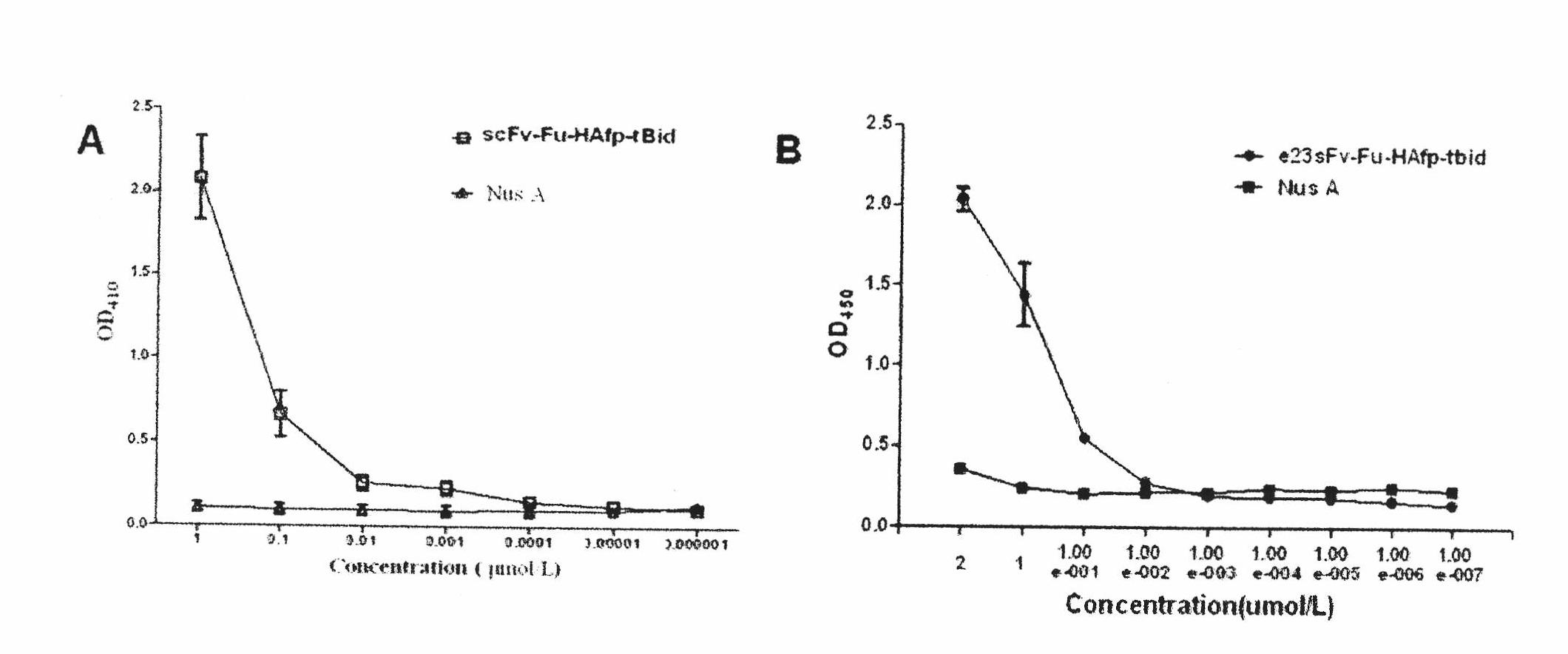
[0045] The pET44b-scFv-Fu-HAfp-tBid plasmid and the pET32a-e23sFv-TD-tBid plasmid were digested with NcoI and EcoRI, and the pET44b-e23sFv-Fu-HAfp-tBid plasmid was obtained by fragment recovery and ligation, and the vector construction was confirmed by enzyme digestion correct (attached figure 1 ), the sequencing results show that the recombinant molecule has the sequence of influenza virus hemagglutinin fusion peptide.
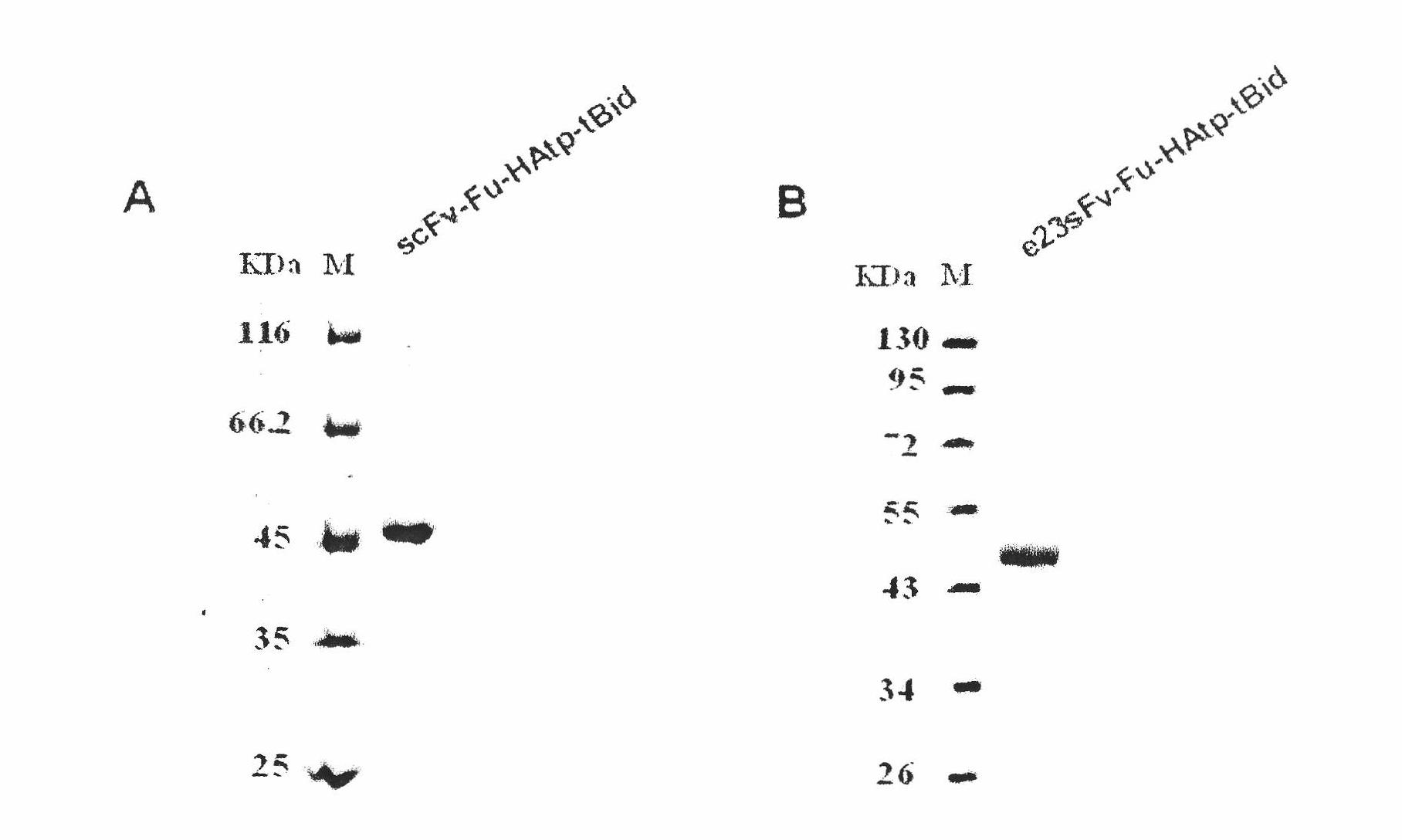
[0046] 3. Prokaryotic expression and product purification of HBsAg / HER2 targeting pro-apoptotic molecules
[0047] The successfully constructed pET44b-scFv-Fu-HAFP-tBid plasmid and pET44b-e23sFv-Fu-HAfp-tBid were transformed into Escherichia coli protein expression host strain RosettaBlue (DE3), single clones were picked, and inoculated in a medium containing ampicillin (100 μg / mL), tetracycline (12.5 μg / mL), chloramphenicol (34 μg / mL) in the LB medium, 37 ° C, 220 rpm shaking overnig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com