Application of three biflavone monomer components extracted from ginkgo leaves in preparing medicament of alpha-glucosidase inhibitor
A technology of ginkgo biflavonoids and glucosidase, which is applied in the field of medicine and can solve the problems of undiscovered ginkgo biflavonoids and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
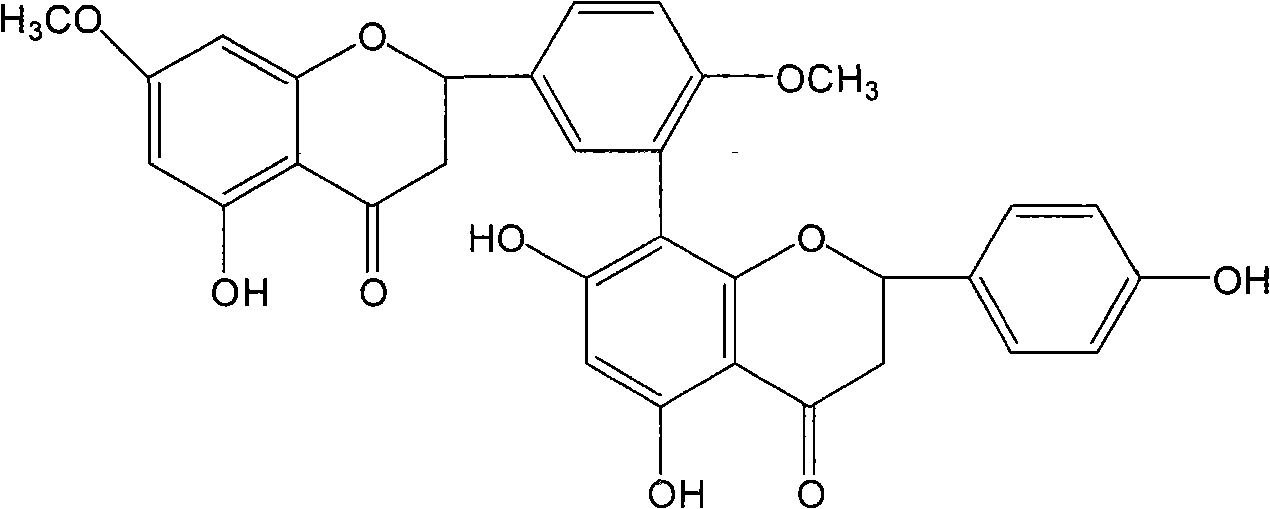
[0051] Example 1 , Preparation of Ginkgo Biflavone, Iso-Ginkgo Biflavone and 7-Demethyl Ginkgo Biflavone
[0052] Take 1kg of dried ginkgo leaves, add 8000ml of 90°C water to soak for 3 times, each time for 3 hours, filter, and discard the filtrate. Add 8000ml of 95% ethanol to the soaked ginkgo leaves for 3 hours, then add 6000ml of 75% ethanol to extract twice, each time for 2 hours, filter, combine the filtrates, directly pass through the treated macroporous resin column, and use 75% Elute 3BV with ethanol, collect the effluent and eluate, recover ethanol, dry, add 250ml of petroleum ether for ultrasonic extraction 3 times, filter, discard the petroleum ether, dissolve the filter residue with 80% ethanol, recrystallize, and dry to obtain the total Biflavones. Get the total biflavonoids of Ginkgo biloba, dissolve them with methanol-0.1% acetic acid aqueous solution (70:30), separate and purify them on a C-18 reverse-phase column (atmospheric pressure or medium and low pres...
Embodiment 2
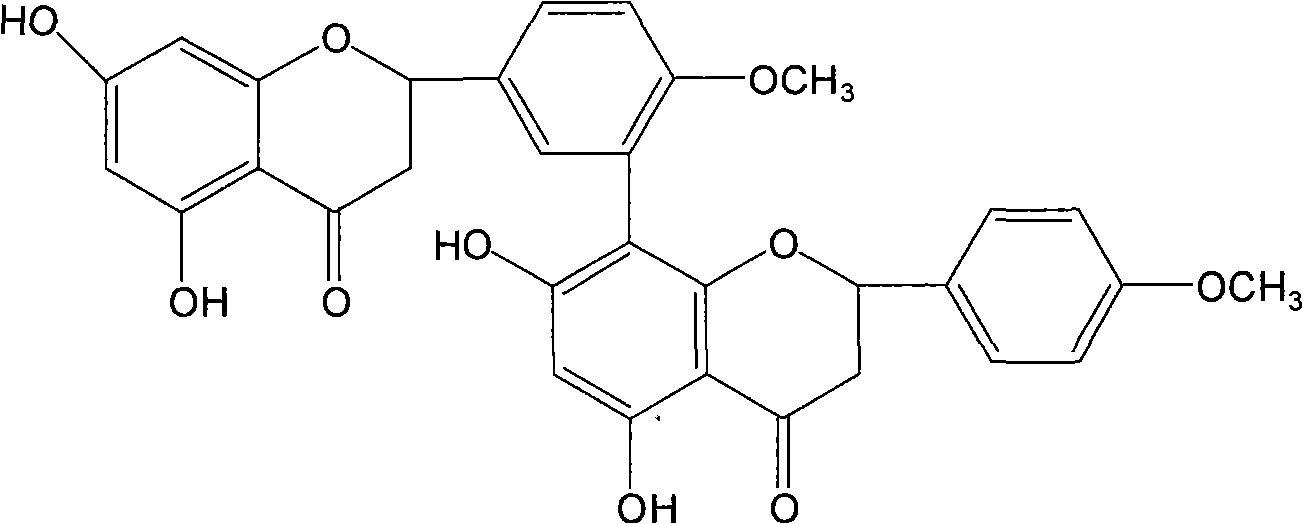
[0053] Example 2 , Preparation of Ginkgo Biflavone, Iso-Ginkgo Biflavone and 7-Demethyl Ginkgo Biflavone
[0054] Take 1kg of dried ginkgo leaves, add 8000ml of 90°C water to soak for 3 times, each time for 3 hours, filter, and discard the filtrate. Ginkgo biloba leaves soaked in water were dried, and 8 times the amount of 75% ethanol was added to reflux 3 times, each time for 3 hours, filtered, and the filtrate was combined, directly passed through the treated macroporous resin column, and 4BV was eluted with 75% ethanol, collected The effluent and the eluate were recovered with ethanol, dried, added 300ml of petroleum ether and ultrasonically extracted 3 times, filtered, discarded the petroleum ether liquid, dissolved the filter residue with 80% ethanol, recrystallized, and dried to obtain the total biflavonoids of Ginkgo biloba leaves. Get the total biflavonoids of Ginkgo biloba, dissolve them with methanol-0.1% acetic acid aqueous solution (70:30), separate and purify th...
Embodiment 3
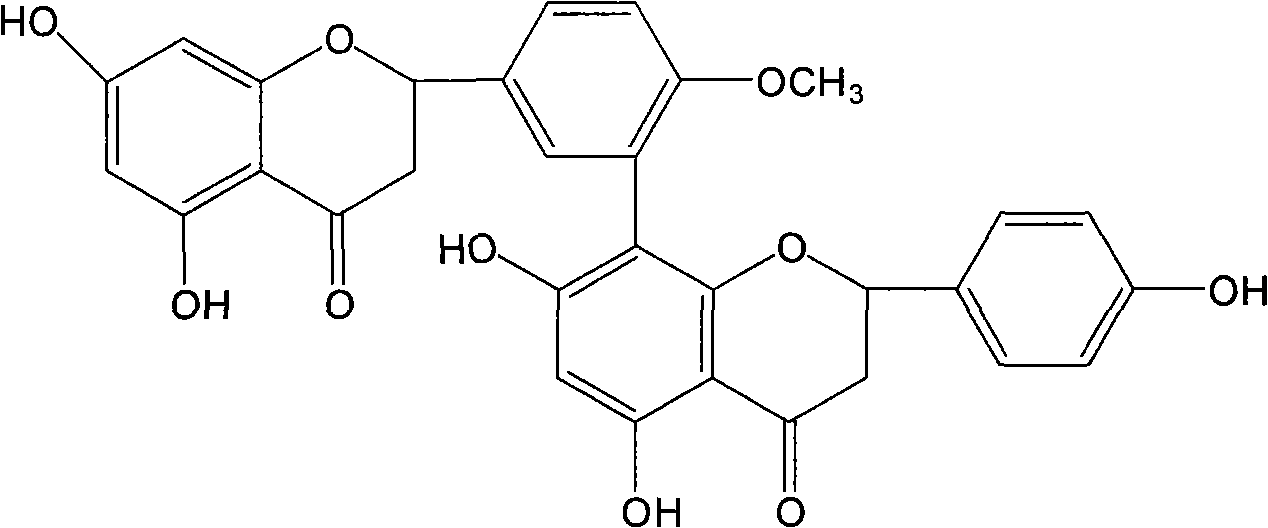
[0055] Example 3 , Preparation of Ginkgo Biflavone, Iso-Ginkgo Biflavone and 7-Demethyl Ginkgo Biflavone
[0056] Take 1kg of dried ginkgo leaves, add 8000ml of 60°C water to soak for 3 times, each time for 3 hours, filter, and discard the filtrate. The ginkgo leaves soaked in water were dried, and 8 times the amount of 80% ethanol was added to reflux 3 times, each time for 3 hours, filtered, and the filtrate was combined, directly passed through the polyamide resin column that had been treated, and 3BV was eluted with 80% ethanol, and collected The effluent and the eluate were recovered with ethanol, dried, added 500ml of petroleum ether and ultrasonically extracted 3 times, filtered, discarded the petroleum ether liquid, dissolved the filter residue with 90% ethanol, recrystallized, and dried to obtain the total biflavonoids of Ginkgo biloba leaves. Get the total biflavonoids of Ginkgo biloba, dissolve them with methanol-0.1% acetic acid aqueous solution (70:30), separate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com