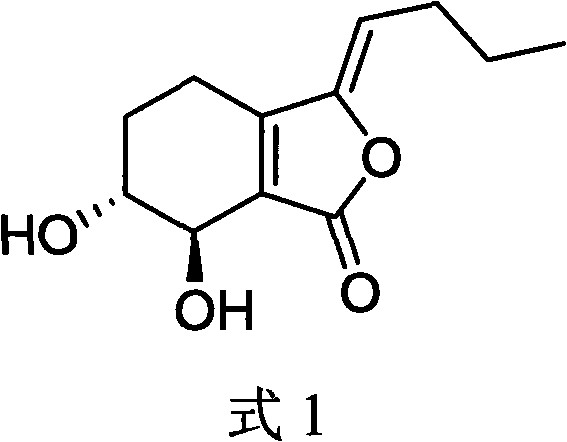
Application of Ligusticolide I in the preparation of antidepressant drugs, migraine drugs and other serotonergic system-related disease drugs
A technology of liguscaridone and migraine, which is applied in the field of new medical application of lisanthemum lactone I, and can solve problems such as reduction and reduction of aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 (alcohol extraction method chromatographic preparation of Ligustilide I)
[0015] Chuanxiong decoction pieces 1kg, after removing impurities, crushed into powder, 70% ethanol 10L reflux extraction twice, 2 hours each time, combined extracts, recovered solvent to obtain about 100g of extract A. The extract was separated by silica gel column chromatography (F100*1000mm). Chloroform-methanol was eluted at 50:1, the 4th-6th BV fraction was collected, and the recovered solvent was purified by a gel column (sephedex LH-20, F20*1500mm), eluted with methanol, and the samples were collected by pointing the plate to obtain purified Ligusticolactone I.
Embodiment 2
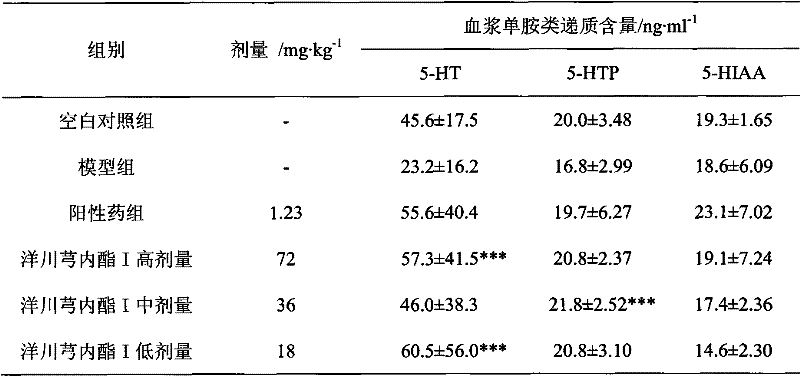
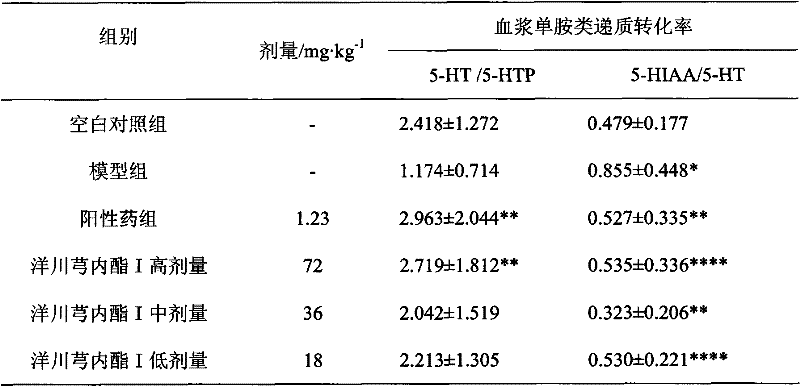
[0016] Embodiment 2 (the effect of Liguscaractone I on rat plasma and brain tissue monoamine transmitters)
[0017] 60 SD rats, half male and half female, body weight (250 ± 20) g, were randomly divided into 6 groups: blank control group, model group, positive drug group, ligustalactone I high, medium and low dose groups. Fasted for 12 h before the experiment, and had free access to water. Modeling method: In addition to the normal group, subcutaneous injection of absolute ethanol 4ml·kg -1 In addition, the other groups were subcutaneously injected with nitroglycerin 10mg / kg. Administration was given 30 minutes after modeling. The positive drug group was given 1.23mg·kg -1 The aqueous solution of ergotamine and caffeine tablets; the high, medium and low dose groups of Ligustilide I were given 72mg·kg by intragastric administration respectively. -1 、36mg·kg -1 , 18mg·kg -1 Ligusticolide I aqueous solution, and the other groups were intragastrically administered equal volu...
Embodiment 3
[0033] Embodiment 3 (the impact of Liguscaractone I on rat plasma and brain tissue NO)
[0034] Fifteen SD rats, male, weighing (250±20) g, were randomly divided into 3 groups: blank control group, model group, and administration group. Fasted for 12 h before the experiment, and had free access to water. Modeling method: In addition to the blank control group, subcutaneous injection of absolute ethanol 4ml kg -1 In addition, the model group and the model administration group were subcutaneously injected with nitroglycerin 10 mg kg -1 . Administration was given 30 minutes after modeling. The administration group was given 72mg·kg by intragastric administration -1 Ligusticolide I aqueous solution, and the other groups were intragastrically administered equal volumes of distilled water. 4 hours after administration, 6ml of blood was collected from the abdominal aorta, injected into a heparinized centrifuge tube, mixed evenly, 4000r·min -1 Centrifuge for 10 minutes to separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com