Fischer-Tropsch synthesis method for heavy hydrocarbon
A technology for Fischer-Tropsch synthesis and heavy hydrocarbons, which is applied in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, metal/metal oxide/metal hydroxide catalysts, etc. Response heat removal is difficult and other problems, to achieve the effect of promoting dispersion, improving activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 5 grams of water to 1.85 grams of potassium hydroxide and dissolve after heating to obtain material (A); 371.7 grams of ferric nitrate and 55.0 grams of copper nitrate are dissolved in 450 grams of hot water at 60 to 90°C to obtain material (B); Lanthanum nitrate, add 40 grams of water, heat and dissolve as material (C).
[0031] Material (A) is mixed with 687.5 grams of silica sol with a weight concentration of 40%, and materials (B) and (C) are added successively under stirring, and the acidity of the above-mentioned slurry is adjusted with ammonia water so that the pH of the mixed slurry=6.0, after After fully stirring, slurry is obtained, and the slurry made is carried out into microspheres in a spray drier according to the usual method, and the inner diameter is 89 millimeters at last, and the length is 1700 millimeters ( mm) in a rotary roaster at 500°C for 2.0 hours to make the composition:
[0032] 40% Fe 100 K 3.0 La 6.0 Cu 25.0 o x +60% SiO2 2
[...
Embodiment 2~4
[0035] Adopt the method substantially identical with embodiment 1 to prepare the catalyst with different composition, gained catalyst number and composition are respectively:
[0036] 1 40%Fe 100 K 3.0 La 6.0 Cu 25.0 o x +60% SiO2 2
[0037] 2 45% Fe100 K 2.0 La 3.0 Ce 2.5 Cu 20.0 o x +55%Al 2 o 3
[0038] 3 50%Fe 100 K 1.0 Cs 3.0 Ce 4.5 Cu 10.0 mn 8.0 o x +50% SiO2 2
[0039] 4 50% Fe 100 Ca 4.5 Ce 3.0 Cu 45.0 o x +50% (70% SiO 2 +30%Al 2 o 3 )
[0040] 5 55% Fe 100 Ca 3.0 Mg 3.0 La 2.0 mn 30.0 o x +45%SiO2 2
[0041] 6 60% Fe 100 Na 2.0 Mg 3.0 Cu 50.0 o x +40% SiO2 2
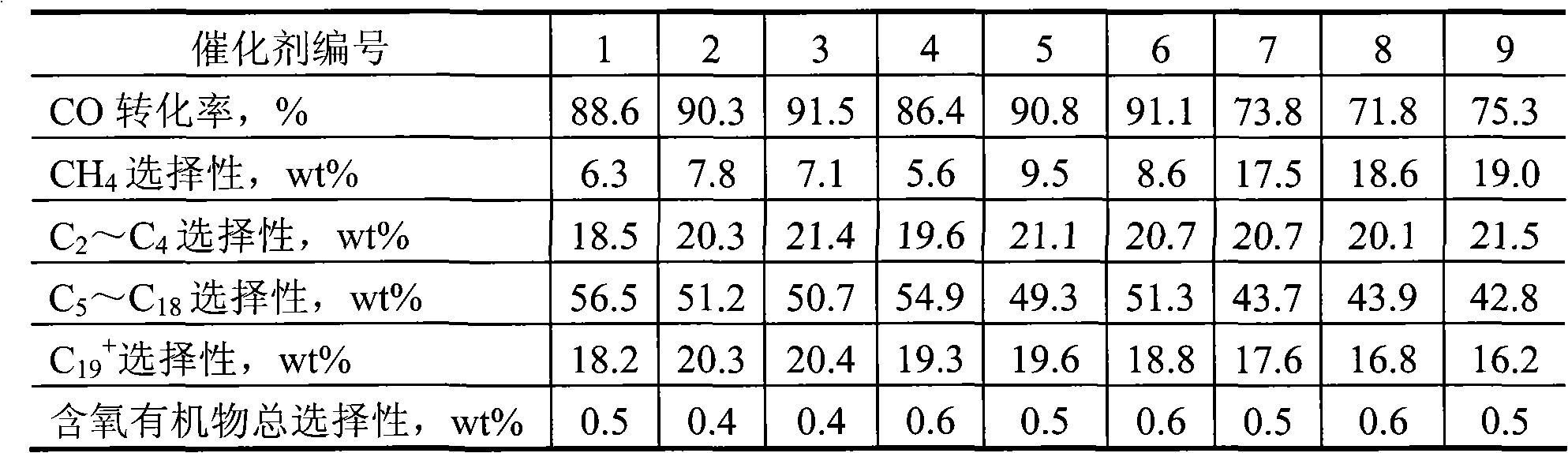
[0042] The prepared catalyst was subjected to Fischer-Tropsch synthesis reaction under the following reaction conditions, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com