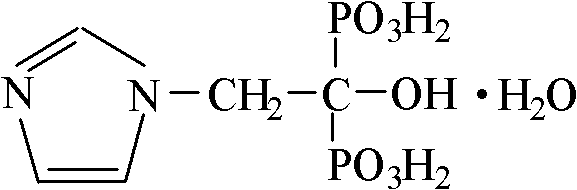Zoledronic acid composition for injection and preparation method thereof
A technology of zoledronic acid and its composition, which is applied in the field of zoledronic acid composition and its preparation, can solve problems such as unresolved technical defects, and achieve avoidance of scrapping, good uniformity, elimination of quality differences between batches and decarbonization The effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
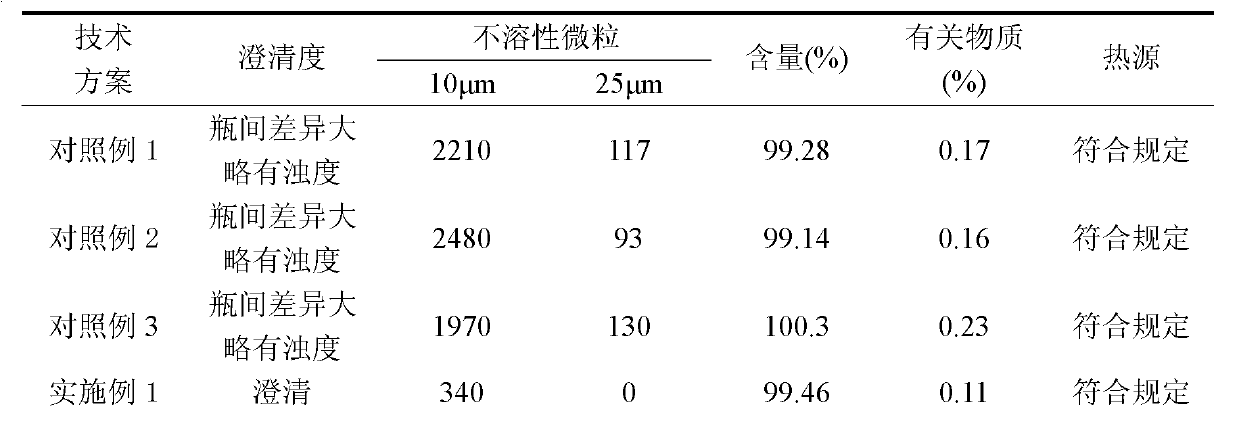
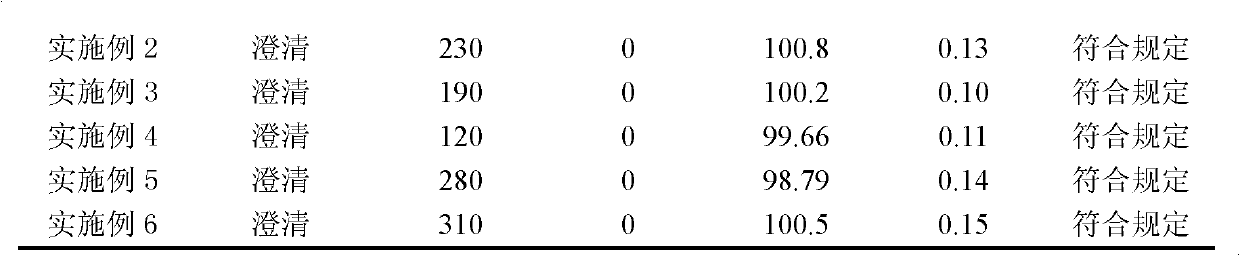
Embodiment 1
[0066] Example 1 Preparation of zoledronic acid compositions for injection
[0067] prescription:
[0068] Zoledronic acid 80g
[0069] Sodium citrate 40g
[0070] Add water for injection to 20Kg
[0071] 1) Preparation of medicinal solution: Weigh zoledronic acid and citrate sodium salt and place in a preparation tank, add water for injection to 20Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 4.5;
[0072] 2) Activated carbon finishing:
[0073] a. Weigh 1g of activated carbon, add 500g of water for injection, stir to disperse evenly, adjust the pH value of the solution to 4.5 with 1% sodium citrate solution, heat to 60°C and keep it warm for 30 minutes, and transport the activated carbon suspension while it is hot To titanium rod filter decarbonization;
[0074] B, then rinse the activated carbon collected by the titanium rod filter with water for injection until the pH value is greater than 5;
[0075] c. Use 0.1% ethylenediaminetetra...
Embodiment 2
[0080] Example 2 Preparation of zoledronic acid compositions for injection
[0081] prescription:
[0082] Zoledronic acid 80g
[0083] Sodium citrate 80g
[0084] Mannitol 1Kg
[0085] Add water for injection to 100Kg
[0086] 1) Preparation of medicinal solution: Weigh zoledronic acid, sodium citrate and mannitol and place in a preparation tank, add water for injection to 100Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 5.0;
[0087] 2) Activated carbon finishing:
[0088] a. Weigh 5g of activated carbon, add 2.5Kg of water for injection, stir to disperse evenly, adjust the pH value of the solution to 5.0 with 1% sodium citrate solution, heat to 65°C and keep it warm for 30 minutes, and dissolve the activated carbon suspension while it is hot Transport to titanium rod filter for decarbonization;
[0089] B, then rinse the activated carbon collected by the titanium rod filter with water for injection until the pH value is greater than 5...
Embodiment 3
[0095] Example 3 Preparation of zoledronic acid compositions for injection
[0096] prescription:
[0097] Zoledronic acid 80g
[0098] Sodium citrate 160g
[0099] Lactose 500g
[0100] Add water for injection to 40Kg
[0101] 1) Preparation of medicinal solution: Weigh zoledronic acid, sodium citrate and lactose and place in a preparation tank, add water for injection to 40Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 6.0;
[0102] 2) Activated carbon finishing:
[0103] a. Weigh 4g of activated carbon, add 2Kg of water for injection, stir to disperse evenly, adjust the pH value of the solution to 6.0 with 1% sodium citrate solution, heat to 70°C and keep it warm for 30 minutes, and transport the activated carbon suspension while it is hot To titanium rod filter decarbonization;
[0104] B, then rinse the activated carbon collected by the titanium rod filter with water for injection until the pH value is greater than 5;
[0105] c. Us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com