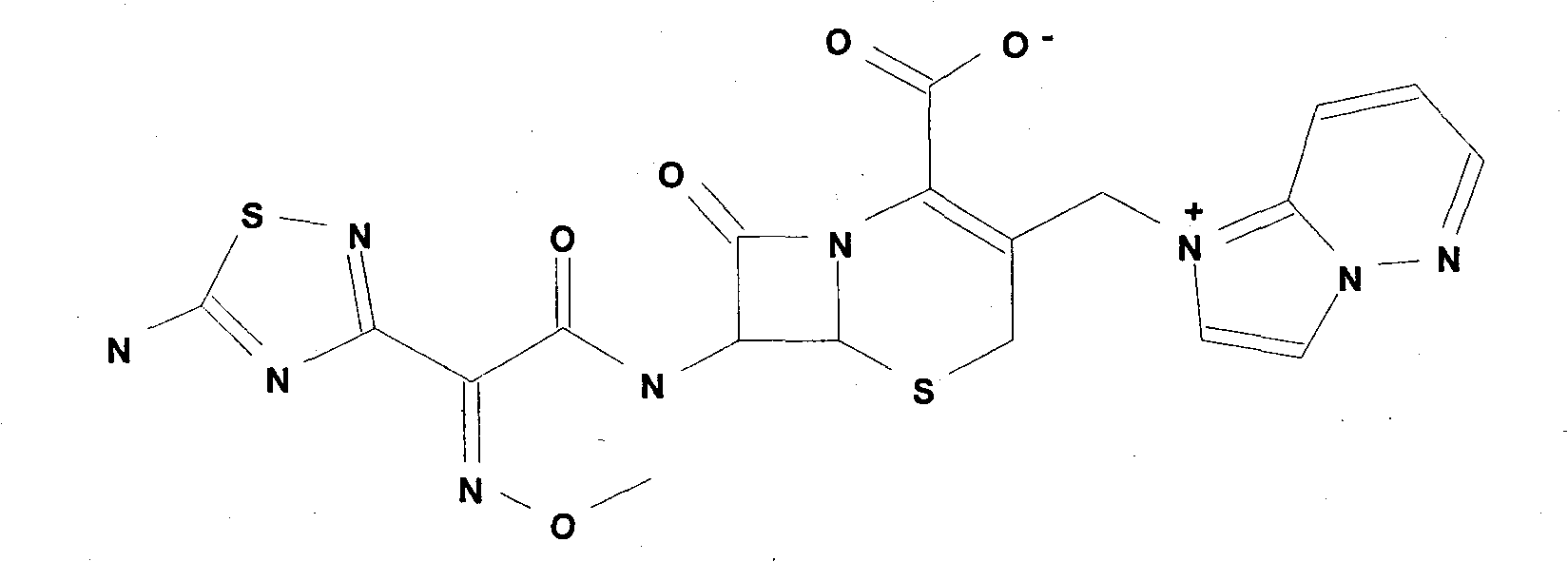
Cefozopran hydrochloride for injection and preparation method thereof
A technology for cefozopran hydrochloride and injection, which is applied in the field of cefozopran hydrochloride for injection and its preparation, can solve the problems of increasing the incidence of adverse reactions, etc., and achieve the effects of reducing dosage, good therapeutic effect, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
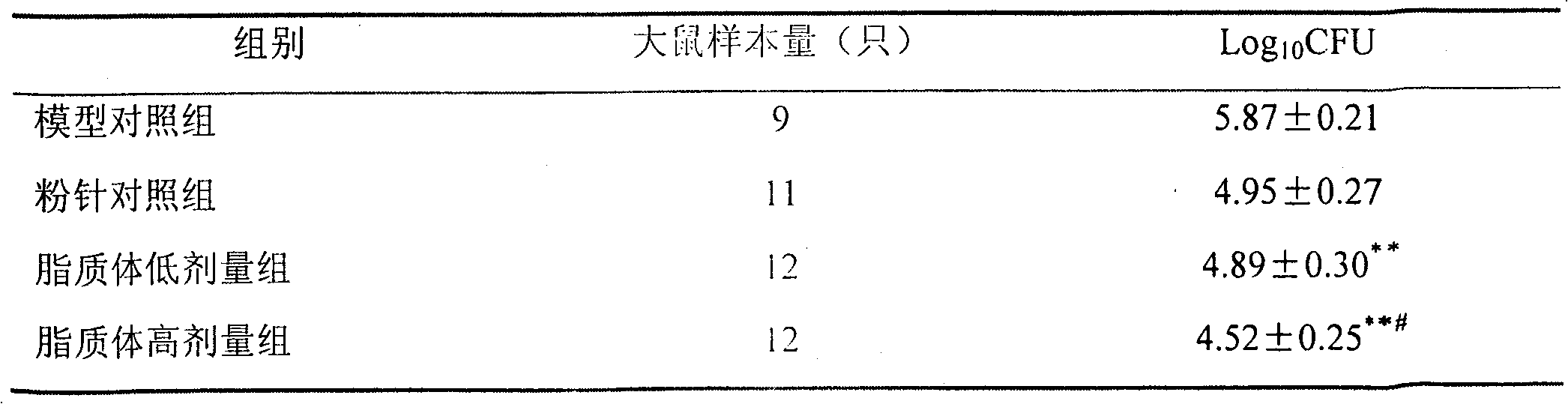
[0065] The preparation of embodiment 1 injection cefozopran hydrochloride
[0066] prescription:
[0067] Cefazolam Hydrochloride 500g
[0068] Soy Lecithin 800g
[0069] Cholesterol 350g
[0070] Anhydrous sodium carbonate 65g
[0071] Glucose 60g
[0072] Preparation Process:
[0073] 1) Take soybean lecithin and cholesterol of prescription quantity, make it dissolve with dehydrated alcohol, mix uniformly;
[0074] 2) Place the liposome liquid prepared in step 1) on a thin wax evaporator, remove ethanol under reduced pressure, and prepare a phospholipid film;
[0075] 3) Immerse an appropriate amount of citric acid-sodium citrate buffer solution with a pH of 4.8 into the phospholipid membrane. After the phospholipid is completely hydrated, prepare liposomes with a high-pressure homogenizer. After checking that the particle size of the liposomes is qualified, microporous filtration Sterilize by membrane filtration, measure the amount of phospholipids with the molybdenu...
Embodiment 2
[0080] The preparation of embodiment 2 injection cefozopran hydrochloride
[0081] prescription:
[0082] Cefazolam Hydrochloride 1000g
[0083] Soy Lecithin 2000g
[0084] Cholesterol 400g
[0085] Anhydrous sodium carbonate 100g
[0086] Glucose 80g
[0087] Preparation process: with embodiment 1
Embodiment 3
[0088] The preparation of embodiment 3 injection cefozopran hydrochloride
[0089] prescription:
[0090] Cefazolam Hydrochloride 200g
[0091] Soy Lecithin 425g
[0092] Soy Lecithin 800g
[0093] Cholesterol 350g
[0094] Anhydrous sodium carbonate 65g
[0095] Glucose 60g
[0096] Preparation Process:
[0097] 1) Take soybean lecithin and cholesterol of prescription quantity, make it dissolve with dehydrated alcohol, mix uniformly;
[0098] 2) Place the liposome liquid prepared in step 1) on a thin wax evaporator, remove ethanol under reduced pressure, and prepare a phospholipid film;
[0099] 3) Immerse an appropriate amount of citric acid-sodium citrate buffer solution with a pH of 4.8 into the phospholipid membrane. After the phospholipid is completely hydrated, prepare liposomes with a high-pressure homogenizer. After checking that the particle size of the liposomes is qualified, microporous filtration Sterilize by membrane filtration, measure the amount of phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com