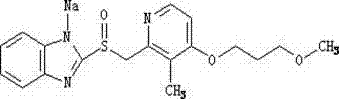
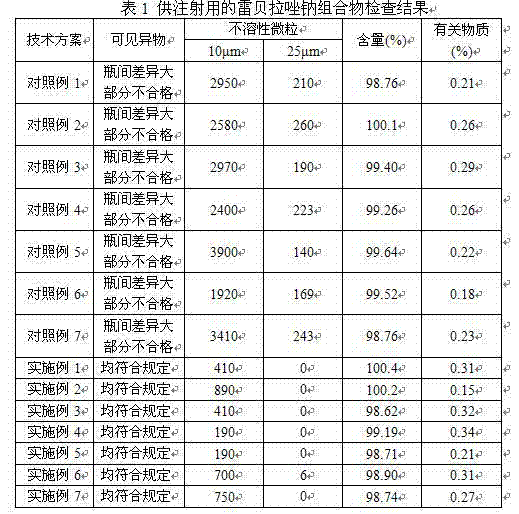
Sodium rabeprazole composition used for injection
A technology of rabeprazole sodium and a composition, which is applied in the field of pharmaceutical preparation, can solve the problems of unsatisfactory barrier effect of inert membrane, unqualified insoluble particles of preparations, and no solution to technical defects, achieves good economic significance, avoids scrap, improves Effect of quality level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Preparation of Rabeprazole Sodium Compositions for Injection
[0087] prescription:
[0088] Rabeprazole Sodium 213g
[0089] EDTA 4.26g
[0090] Add water for injection to 20Kg
[0091] 1) Preparation of liquid medicine: Weigh rabeprazole sodium and disodium edetate into a preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 10.5 with sodium hydroxide;
[0092] 2) Rubber stopper treatment:
[0093] a. Take disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 7.0 with sodium hydroxide after stirring and dissolving;
[0094] b. Take the rubber stopper, add solution a according to the weight ratio of the stopper and solution a of 1:10, heat to 41°C and keep it warm for 30 minutes;
[0095] c. Take the rubber stopper that has been treated in the process of b, rinse it with water for injectio...
Embodiment 2
[0098] Example 2 Preparation of Rabeprazole Sodium Compositions for Injection
[0099] prescription:
[0100] Rabeprazole Sodium 213g
[0101] EDTA 6.39g
[0102] Add water for injection to 20Kg
[0103] 1) Preparation of medicinal solution: Weigh rabeprazole sodium and disodium edetate into a preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 11.5 with sodium hydroxide;
[0104] 2) Rubber stopper treatment:
[0105] a. Get disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 6.0 with sodium hydroxide after stirring and dissolving;
[0106] b. Take the rubber stopper, add solution a according to the weight ratio of the stopper and solution a of 1:10, heat to 41°C and keep it warm for 30 minutes;
[0107] c. Take the rubber stopper that has been treated in the process of b, rinse it with water for inject...
Embodiment 3
[0110] Example 3 Preparation of Rabeprazole Sodium Compositions for Injection
[0111] prescription:
[0112] Rabeprazole Sodium 213g
[0113] EDTA 8.52g
[0114] Add water for injection to 20Kg
[0115] 1) Preparation of medicinal solution: Weigh rabeprazole sodium and disodium edetate into a preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 11.0 with sodium hydroxide;
[0116] 2) Rubber stopper treatment:
[0117] a. Get disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 6.5 with sodium hydroxide after stirring and dissolving;
[0118] b. Take the rubber plug, add solution a according to the weight ratio of rubber plug and solution a 1:10, heat to 45°C and keep it warm for 30 minutes;
[0119]c. Take the rubber stopper that has been treated in the process of b, rinse it with water for injection fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com