Solution for surgery and endoscope washing and preparation method thereof
A solution and laparoscopic technology, applied in the direction of surgical drugs, pharmaceutical formulations, drug delivery, etc., can solve problems such as cartilage softening, collagen swelling, iatrogenic damage to articular cartilage, etc., to achieve suitable transportation, ensure stability, and guarantee safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Solution
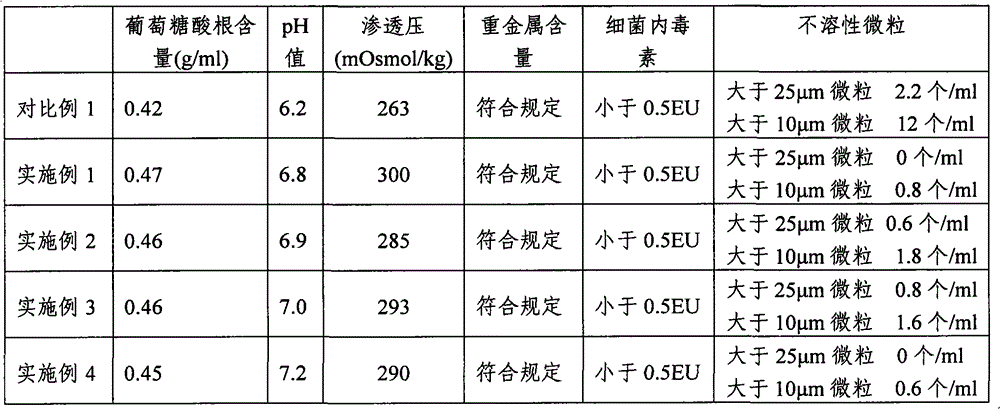
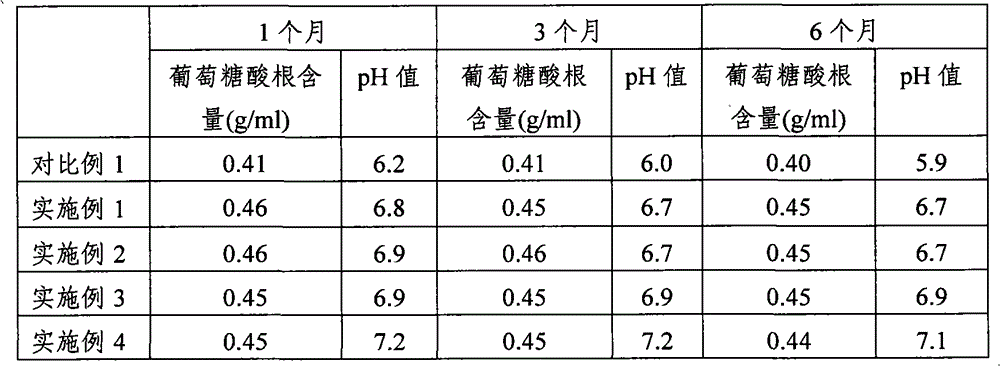
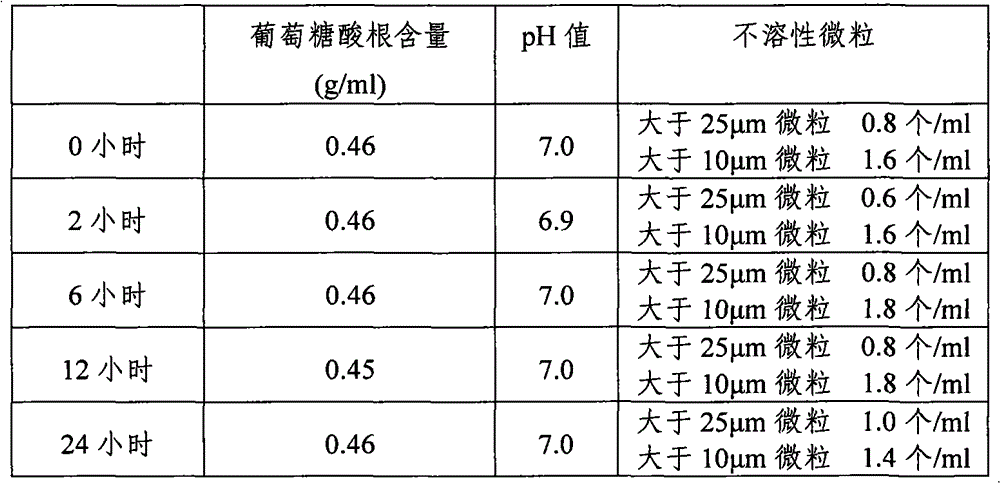
[0031] 1. Raw materials: sodium chloride (NaCl) 526g, sodium acetate (C 2 h 3 NaO 2 ·3H 2 O) 368g, sodium gluconate (C 6 h 11 ·NaO 7 ) 502g, potassium chloride (KCl) 37g and magnesium chloride (MgCl 2 ·6H 2 O) 30g.
[0032] 2. Preparation method: Add 20 liters of water for injection into the concentrated preparation tank, weigh the prescribed amount of sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride, put them in the concentrated preparation tank, and put them in after stirring and dissolving 0.1% (g / mL) activated carbon for needles, boil for 15 minutes, stir well, let it stand for adsorption for 15 minutes, cool to 60°C, pass through a 5μm titanium rod for decarbonization and circulation for 15 minutes, and then inject it through a titanium rod and a 0.45μm filter element Dilute cans. Add water for injection to 100 liters in the dilute preparation tank, stir and circulate for 15 minutes, adjust...
Embodiment 2
[0033] Example 2: Solution
[0034] 1. Raw materials: sodium chloride (NaCl) 526g, sodium acetate (C 2 h 3 NaO 2 ·3H 2O) 368g, sodium gluconate (C 6 h 11 ·NaO 7 ) 502g, potassium chloride (KCl) 37g and magnesium chloride (MgCl 2 ·6H 2 O) 30g.
[0035] 2. Preparation method: Add 20 liters of water for injection into the concentrated preparation tank, weigh the prescribed amount of sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride, put them in the concentrated preparation tank, and put them in after stirring and dissolving 0.1% (g / mL) activated carbon for needles, boil for 15 minutes, stir well, let it stand for adsorption for 15 minutes, cool to 60°C, pass through a 5μm titanium rod for decarbonization and circulation for 15 minutes, and then inject it through a titanium rod and a 0.45μm filter element Dilute cans. Add water for injection to 100 liters in the dilute preparation tank, stir and circulate for 20 minutes, adjust ...
Embodiment 3
[0036] Example 3: Solution
[0037] 1. Raw materials: sodium chloride (NaCl) 526g, sodium acetate (C 2 h 3 NaO 2 ·3H 2 O) 368g, sodium gluconate (C 6 h 11 ·NaO 7 ) 502g, potassium chloride (KCl) 37g and magnesium chloride (MgCl 2 ·6H 2 O) 30g.
[0038] 2. Preparation method: Add 20 liters of water for injection into the concentrated preparation tank, weigh the prescribed amount of sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride, put them in the concentrated preparation tank, and put them into the concentrated preparation tank after stirring and dissolving 0.1% (g / mL) activated carbon for needles, boil for 15 minutes, stir well, let it stand for adsorption for 15 minutes, cool to 60°C, pass through a 5μm titanium rod for decarbonization and circulation for 15 minutes, and then inject it through a titanium rod and a 0.45μm filter element Dilute cans. Add water for injection to 100 liters in the dilute preparation tank, stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com