Antigen and preparation method and application thereof
An antigen and reaction technology, applied in chemical instruments and methods, carrier-binding antigen/hapten components, pharmaceutical formulations, etc., can solve the problems of patients' negative emotions, hinder endogenous opioids, etc., and prevent relapse. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the preparation of morphine-6-glutaric acid monoester-bovine serum albumin antigen (morph-BSA)
[0047] Small molecule (small molecule less than 10KDa) drugs must be coupled to a carrier protein before they can cause an immune response. Therefore, in order to generate an immune response, the synthesis of small molecule drug derivatives, that is, the synthesis of haptens, must first be carried out.
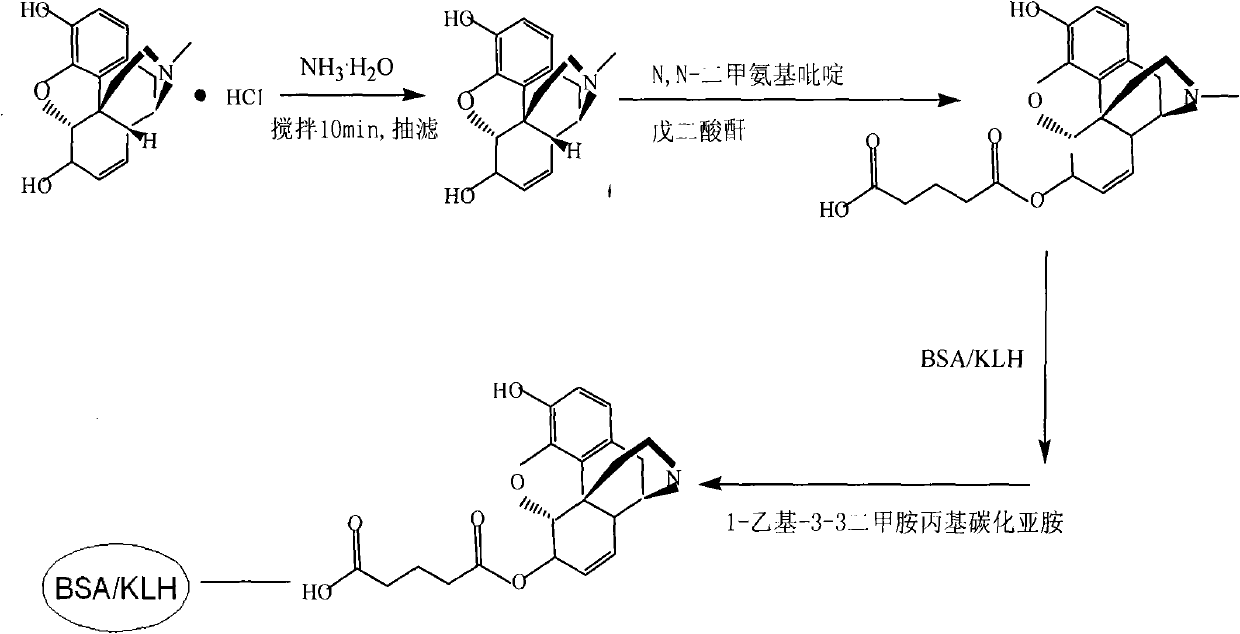
[0048] Antigen synthesis route diagram as figure 1As shown, the specific steps are as follows:
[0049] 1. Preparation of morphine-6-glutaric acid monoester
[0050] (1) Dissolve 1.3g of morphine hydrochloride in 30mL of water, add dropwise ammonia water to pH 8.5, react at a temperature of 37°C with stirring for 10min, filter with suction to obtain an off-white solid, and dry it in vacuum for 24h to obtain 1g of free morphine alkali.
[0051] (2) Mix 1 g of morphine free base and 1.2 g of glutaric anhydride obtained in step (1) with a catalytic amount of N...
Embodiment 2
[0061] Embodiment 2, the preparation of morphine-6-glutaric acid monoester-hemocyanin antigen (morph-KLH)
[0062] Antigen synthesis route diagram as figure 1 As shown, the specific steps are as follows:
[0063] 1. Preparation of morphine-6-glutaric acid monoester
[0064] Same as Step 1 of Experiment 1.
[0065] 2. Coupling of morphine-6-glutaric acid monoester and carrier protein
[0066] (1) Dissolve 100 mg of morphine-6-glutaric acid monoester obtained in step 1 and 20 mg of hemocyanin in TE buffer solution (pH 8.0), and fully oscillate to dissolve to obtain liquid I;
[0067] (2) Take 48mg of 1-ethyl-3-3 dimethylaminopropyl carboimide (1-ethyl-3-(3-dimethylaminopropyl), EDC) and fully dissolve in TE buffer solution (pH 8.0) to obtain II liquid;
[0068] (3) Add liquid I dropwise to liquid II while shaking, and react in a shaker at 37°C for 2 hours to obtain a conjugate formed by covalently linking morphine-6-glutaric acid monoester and hemocyanin; The covalent bond...
Embodiment 3
[0070] Embodiment 3, the application of antigen
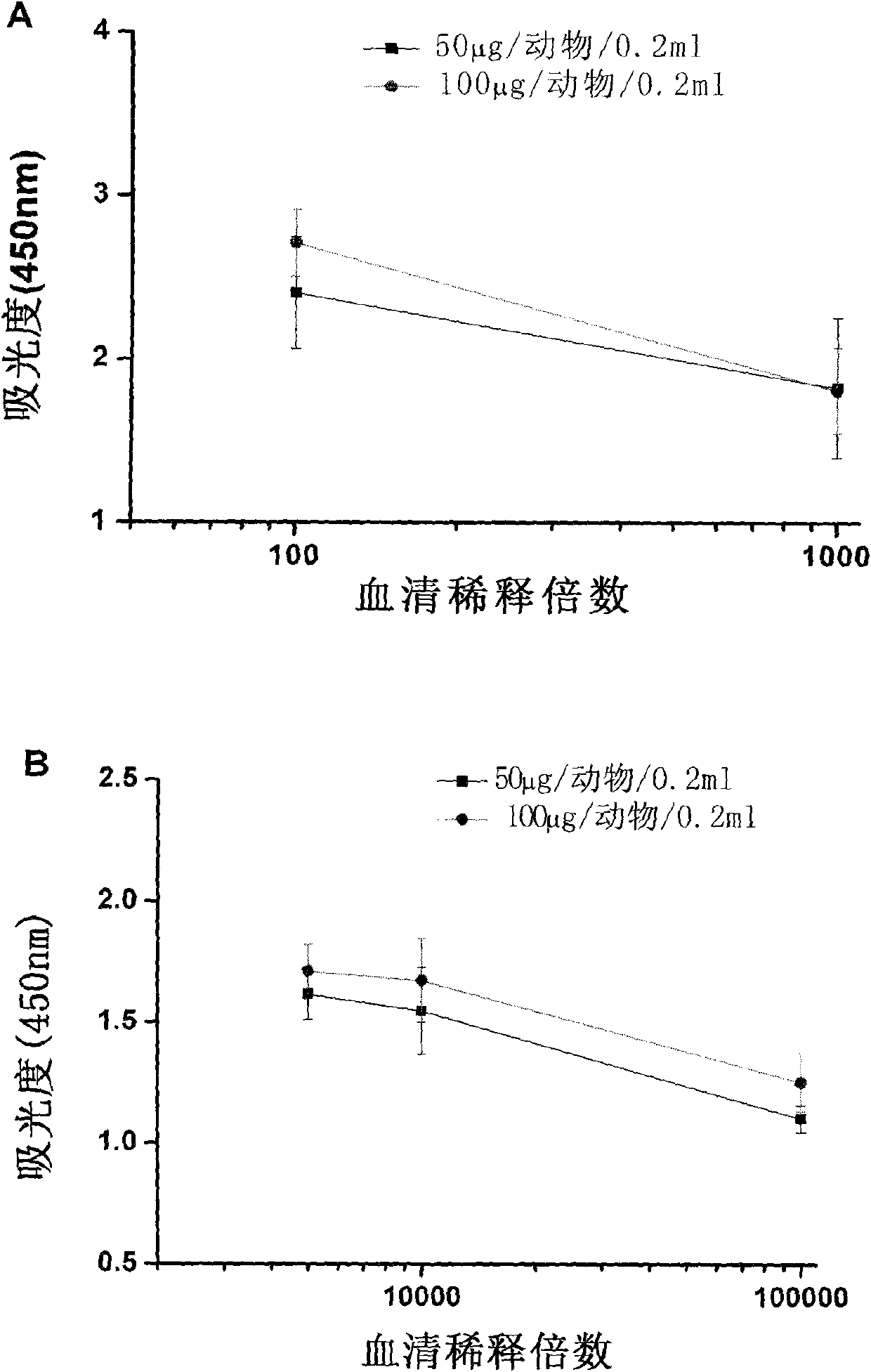
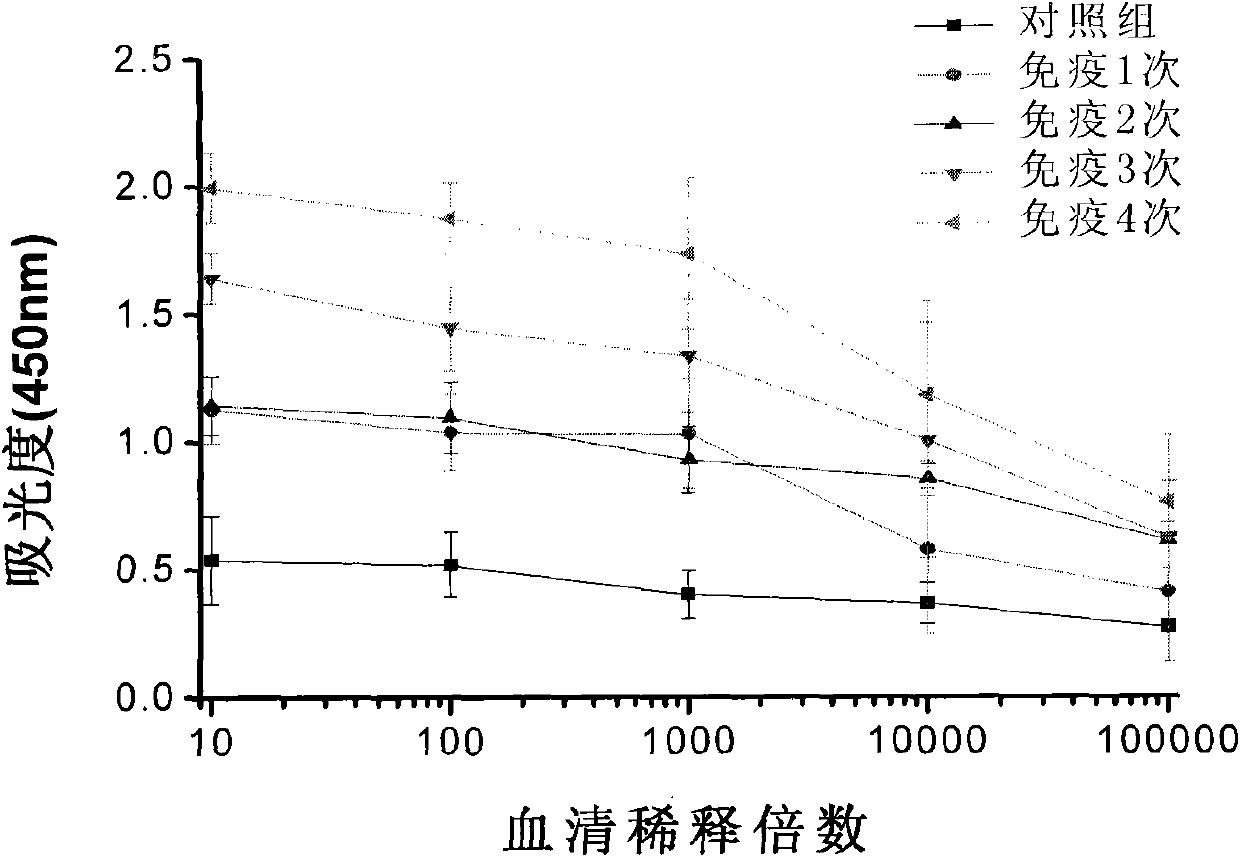
[0071] 1. Actively immunized mice and serum antibody titer analysis
[0072] 1. Prepare antibody using morph-KLH antigen
[0073] Take BABL / C mice (20-25g) as experimental animals. The mice were raised in cages with controllable temperature (21° C.-24° C.) and lights (08:00 on and 20:00 off), free to eat and drink for 5 days, with a body weight of about 25-28 g. Randomly divided into three groups, each group of 8 mice, the three groups of mice were subjected to the following treatments: A, injection of adjuvant+KLH; B, injection of adjuvant+morph-KLH (50 μg / animal / 0.2ml, that is, each Only mice are injected with the mixture of 0.2ml adjuvant and morph-KLH, containing 50 μ g morph-KLH in the 0.2 ml mixture); Adjuvant + moroh-KLH, 0.2ml injection contains 100 μg morph-KLH). The injection method is subcutaneous injection, the adjuvant is Freund's complete adjuvant, and the drug is divided into four injection points on average....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com