Silicia for the inhinition of a protease
A technology of silica and protease, which is applied to medical preparations containing active ingredients, digestive system, pharmaceutical formulas, etc., can solve problems such as silica protease inhibition that is not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0058] Thus, according to one embodiment, the protease of the invention is an aspartic protease.
[0059] In a preferred embodiment, the protease according to the invention is pepsin.
[0060] In a preferred embodiment, the protease according to the invention is mammalian pepsin.
[0061] In a preferred embodiment, the protease according to the invention is selected from the group consisting of human pepsin, porcine pepsin, horse pepsin, mouse pepsin, sheep pepsin, dog pepsin, sheep pepsin and bovine pepsin.
[0062] In a preferred embodiment, the protease of the invention is human gastric pepsin.
[0063] In a preferred embodiment, the protease of the invention is human gastric pepsin. Preferably, the protease according to the invention is a sub-type of human pepsin. Even more preferably, the protease according to the invention is selected from pepsin 1, 3a, 3b, 3c and pepsin.
[0064] In an alternative embodiment, the protease is a serine protease, preferably trypsin. I...
Embodiment
[0110] The present invention will be described in further detail by way of example only with reference to the accompanying drawings, in which
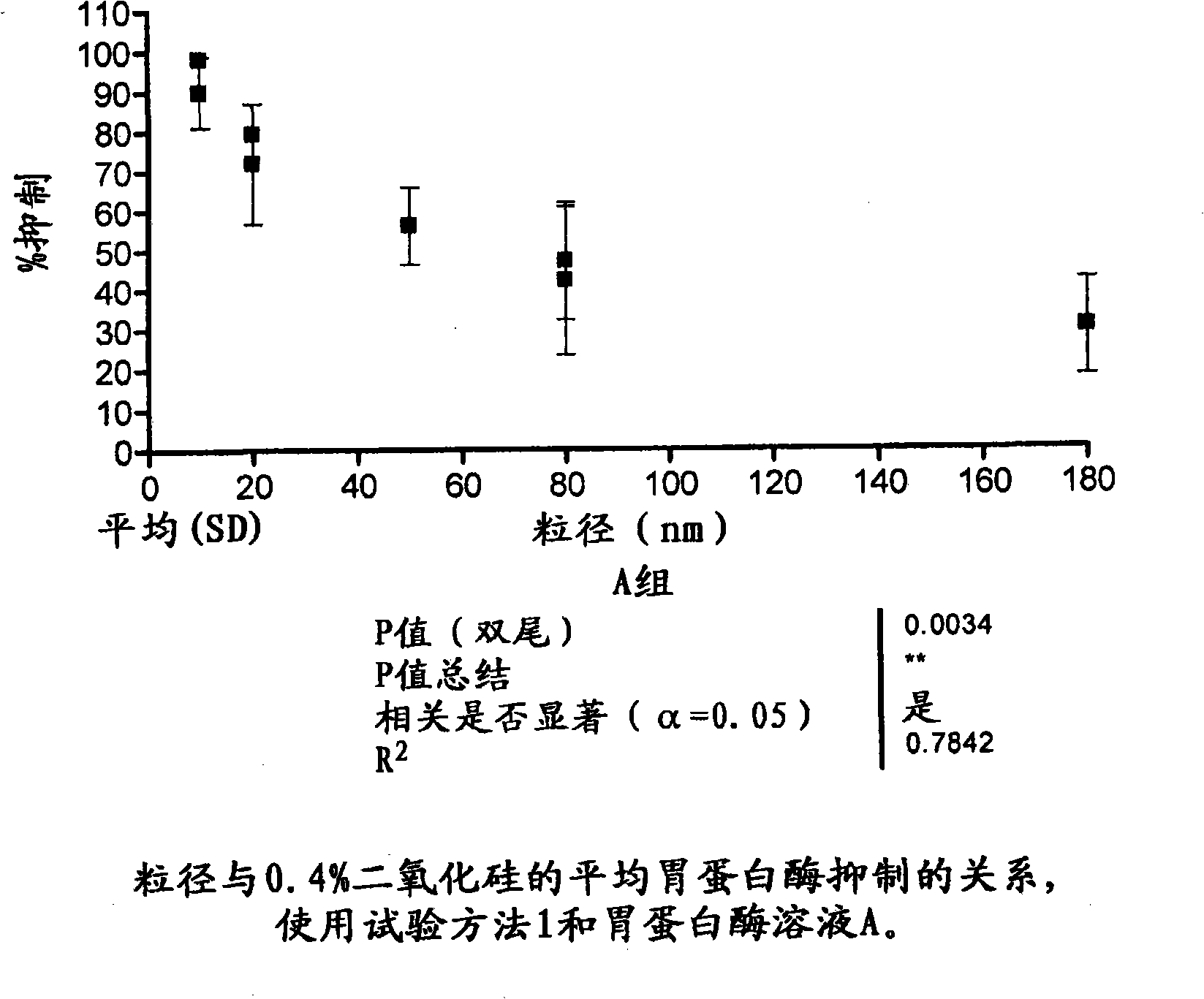
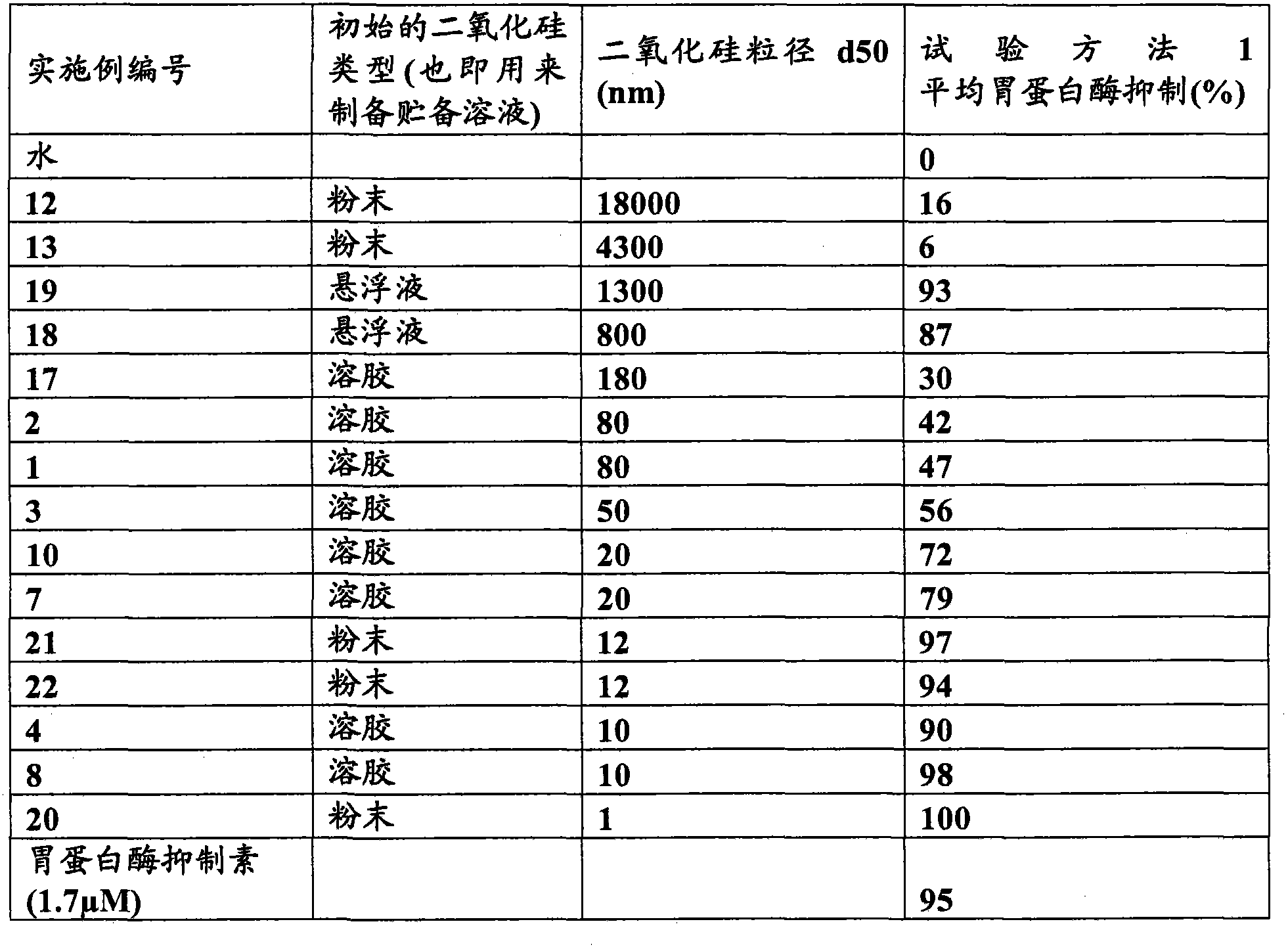
[0111] figure 1 Shown as attached.
[0112] substance
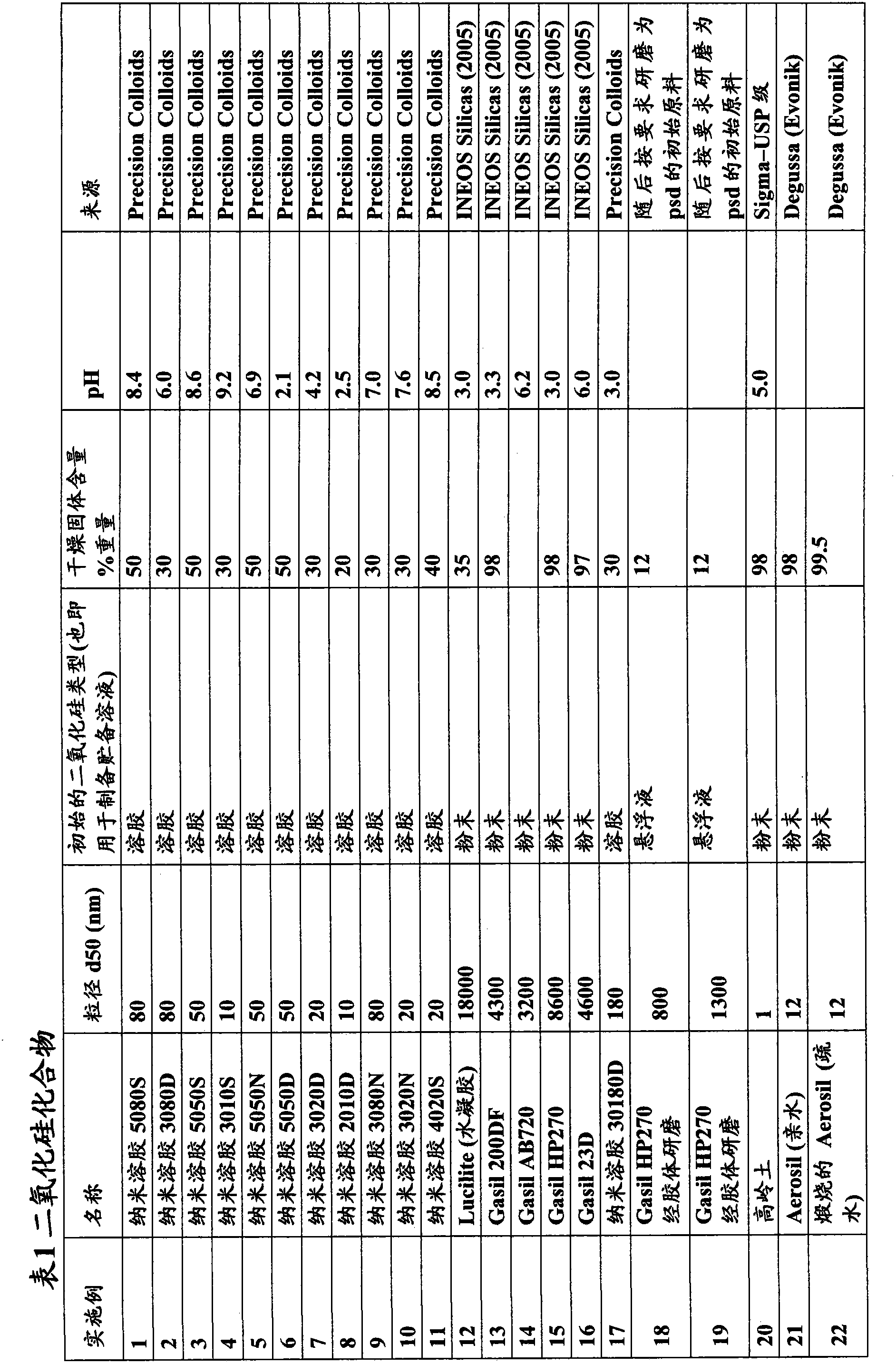
[0113] The silica materials used in this study were obtained from Precision Colloids LLC, Cartersville USA and INEOS Silicas, Warrington UK. Other reagents used were obtained from standard laboratory suppliers.
[0114] Silica material supplied in liquid or sol form is diluted once in deionized water to the desired concentration and shaken well. A volume (as specified below for each test method) is withdrawn from this stock solution to provide the desired final concentration in the test solution (ie, the test solution containing pepsin and / or substrate).
[0115] Disperse the silica material in powder form in deionized water and dilute as needed, shake well. A volume (as specified below for each test method) is withdrawn from this stock solution to provide the desired fina...
preparation Embodiment 18 and 19
[0119] Preparation Examples 18 and 19 - Colloidal Milled Silica:
[0120] The equipment used to colloidally grind Gasil HP270 to the required particle size is as follows:
[0121] ●Eiger Torrance Micro Mill 250
[0122] ●182ml zirconia beads
[0123] The mill was assembled using 182 ml of zirconia beads according to the mill manufacturer's instructions.
[0124] A Gasil HP270 slurry (120 g, 100 ml deionized water) was prepared with 12% w / v solids content and stirred with an overhead paddle stirrer for 10 minutes. The slurry was introduced into the mill and ground for 60 minutes at 4000 rpm. Aliquots were taken every 10 minutes for particle size distribution (PSD) analysis via Malvern Mastersizer to assess milling progress.
[0125] The Malvern Mastersizer method parameters are as follows:
[0126] ●Pump, stirrer and ultrasound set to 50%
[0127] ●2.5 minutes dispersion time
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com