Kit for detecting porcine pseudorabies virus (PRV) and porcine parvovirus (PPV)
A technology for porcine pseudorabies virus and porcine pseudorabies disease, which can be applied in the biological field and can solve problems such as economic losses in the pig industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, establishment of dual PCR system and optimization of conditions
[0034] 1. Primer design and synthesis
[0035] According to the sequence published on GenBank, DNAMan software was used to analyze the homology of PPV VP2 gene (Genbank accession number DQ464345) and PRV gE gene (Genbank accession number AF403049). Multiple pairs of specific primers were designed and identified by NCBI BLAST, all of which had good specificity. Primers were synthesized by Introvigen Shanghai Biotechnology Company. The primer sequences are listed in Table 1.
[0036] Table 1 PPV and PRV double PCR primers
[0037]
[0038]
[0039] 2. Viral nucleic acid extraction
[0040] According to the instructions of the QIAGEN kit
[0041] 1) Add 20 μL proteinase K (proteinase K) into a 1.5 mL sterilized centrifuge tube;
[0042] 2) Add 200 μL each of porcine pseudorabies virus PRV and porcine parvovirus PPV, and mix well. If the volume is less than 200 μL, make up with PBS; ...
Embodiment 2
[0076] Embodiment 2, carry out the detection of clinical sample with double PCR method
[0077] 1. Detection of PPV and PRV in clinical suspected samples
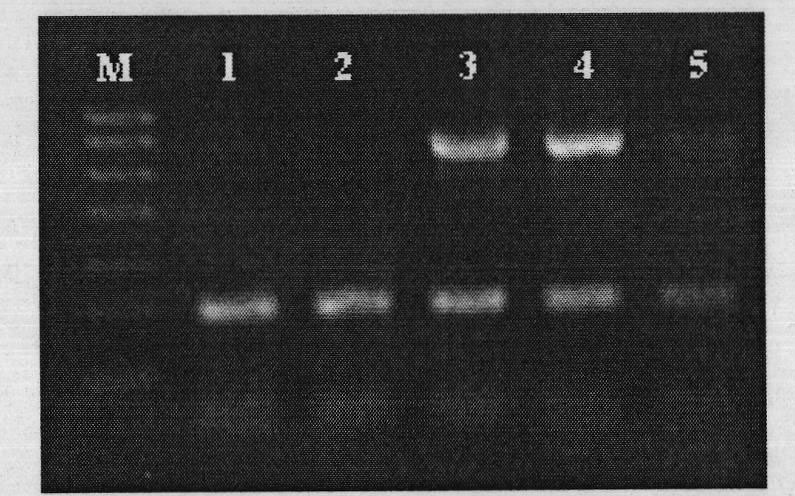
[0078] Tissue samples of suspected diseased pigs from clinically affected pig farms in different regions, please see the following table for sample information: 24 mixed samples including 9 pig brain tissues, 11 pig serum, 4 pig kidneys and lymph nodes, PPV for suspected samples and PRV detection. The mixed DNA of porcine pseudorabies virus PRV and parvovirus PPV was used as a positive control, and sterilized deionized water was used as a negative control. Perform PCR detection according to the optimized double PCR system and double PCR reaction conditions obtained in Example 1.
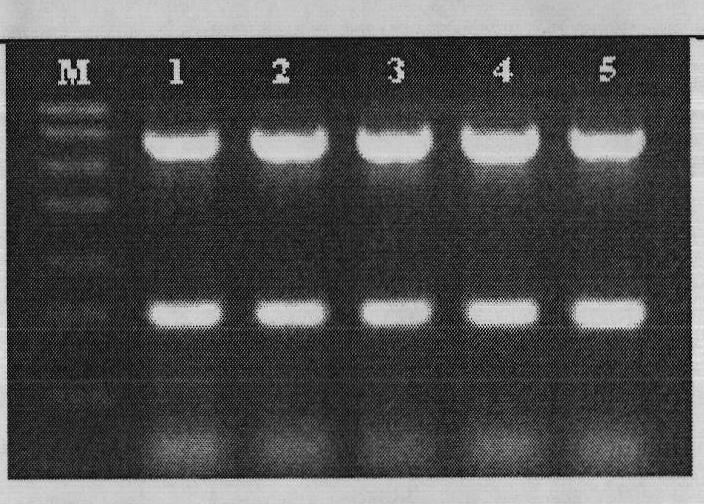
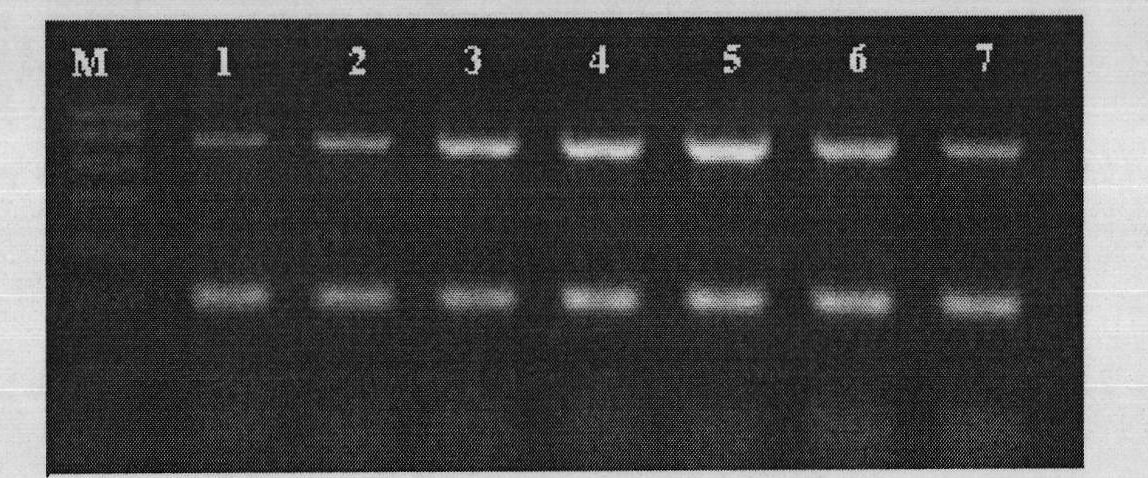
[0079] Test results such as Figure 7 and Figure 8 shown.
[0080] Figure 7 Clinical sample test results 1, M: Trans DNA Marker I, the size from top to bottom is: 700, 600, 500, 400, 300, 200, 100; 1, 2: positive control, negative control, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com