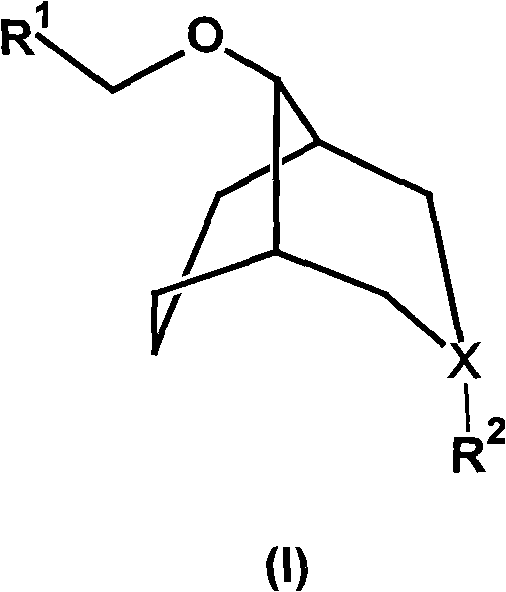
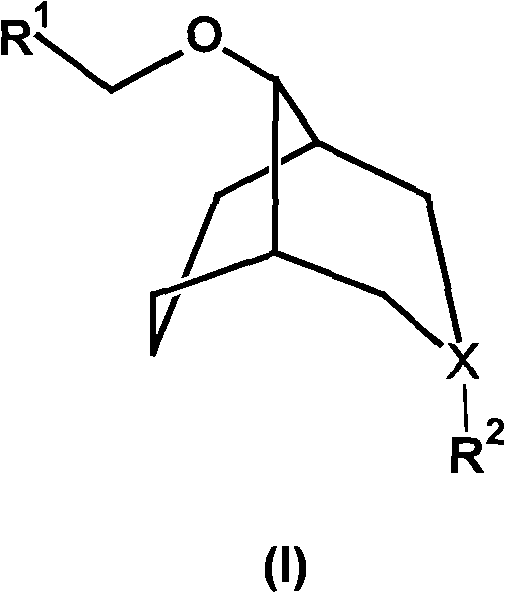
Substituted bicyclo(3,3,1)nonane ether compound and application thereof
A technology of salt compounds and compounds, applied in organic chemistry, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Rac-9-(2'-anthracenyl-2'-naphthyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane (Rac-1 ) preparation
[0051] Under the protection of nitrogen, put 0.6g (15mmol) NaH in a dry three-necked flask, add 10mL of anhydrous DMSO, after stirring for 5min, dropwise add N-methyl-3-azabicyclo(3.3.1) nonanol ( 2.0g (14.2mmol) of 10mL DMSO solution, stirred at 60°C for 1h. Cooled to room temperature, slowly added dropwise 2'-anthracenyl-2'-naphthyl-1,2-oxirane 2.61g (14mmol) 10mL DMSO solution. Stir the reaction at 50°C for 3h, cool, and carefully add 20mL of water dropwise. Extract with ether, wash with water, wash the ether layer with 10% hydrochloric acid solution, and combine the aqueous phase; After drying over sodium sulfate, 9-(2'-anthracenyl-2'-naphthyl-2'-hydroxyl-ethoxyl)-N-methyl-3-azabicyclo (3.3.1 ) nonane colorless liquid, 3.02g, yield 67%. 1 H-NMR: δ (ppm, CD 3 Cl), 7.44(m, 2H), 7.25(m, 3H), 5.02(s, 1H), 3.71-3.85(m, 2H), 2.91-3.05(m, 4H), 2.91(s...
Embodiment 2
[0052] Example 2 Rac-9-(2'-anthracenyl-2'-naphthyl-2'-hydroxy-ethoxy)-N-methyl-3-azabicyclo(3.3.1)nonane hydrochloride ( Preparation of Rac-1·HCl)
[0053]Dissolve 2.0g of (1) in 5mL of ether, cool the ether layer, add 5ml of 2N hydrochloric acid solution, and stir to precipitate a solid. The solid was collected by filtration, washed with ice water, and recrystallized from 95% ethanol to obtain 1.8 g of the title compound as a white solid, yield 81%, melting point: 189-191°C.
[0054] H-NMR: δ (ppm, CD 3 Cl), 10.87(s, 1H), 7.61(m, 2H), 7.25(m, 3H), 5.08(s, 1H), 3.63-3.80(m, 2H), 2.92-3.05(m, 4H), 2.82 (s, 3H), 2.21 (s, 1H), 2.03 (s, 1H), 2.01 (m, 2H), 1.31-1.75 (m, 12H).
Embodiment 3
[0055] Example 3Rac-9-(2'-Anthracenyl-2'-Naphthyl-2'-Hydroxy-ethoxy)-N,N-Dimethyl-3-azabicyclo(3.3.1)nonane quaternary Amiodonium salt (Rac-1-CH 3 I) Preparation
[0056] Dissolve 1.0g (1) and excess methyl iodide in 20mL of acetonitrile, stir and reflux for 5 days, TLC detects that the reaction has reached equilibrium, cool to room temperature, evaporate the solvent, wash the residue 3 times with ethyl acetate, and recrystallize from ethanol , the target compound was obtained as 0.86 g of yellow solid, yield 61%, melting point: 161-163°C. H-NMR: δ (ppm, DMSO), 11.27 (s, 1H), 7.58 (m, 2H), 7.37 (m, 3H), 4.98 (s, 1H), 3.43-3.70 (m, 2H), 2.82- 3.10(m, 4H), 2.88(s, 3H), 2.76(s, 3H), 2.25(s, 1H), 2.06(s, 1H), 2.04(m, 2H), 1.37-1.81(m, 12H) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com