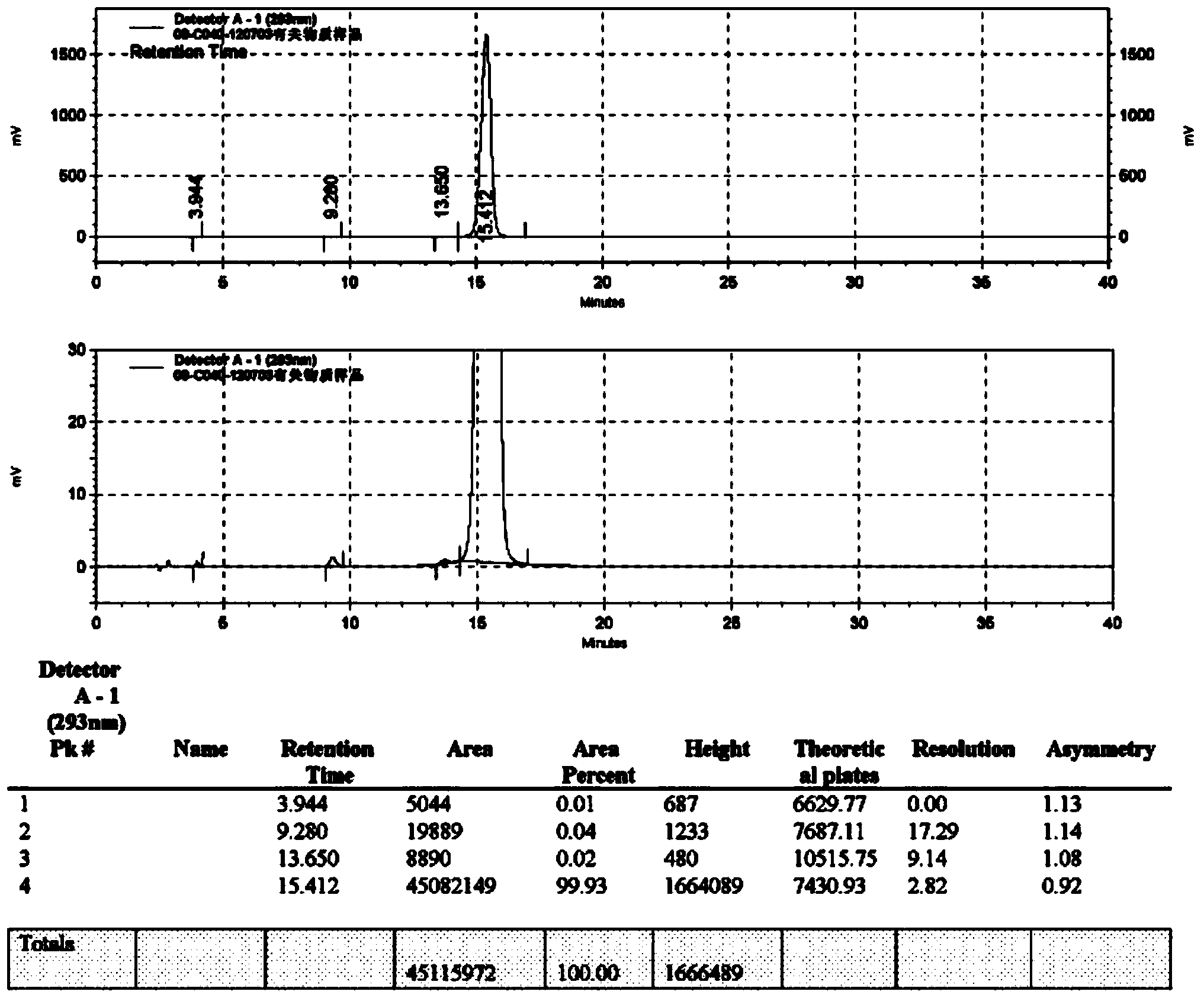
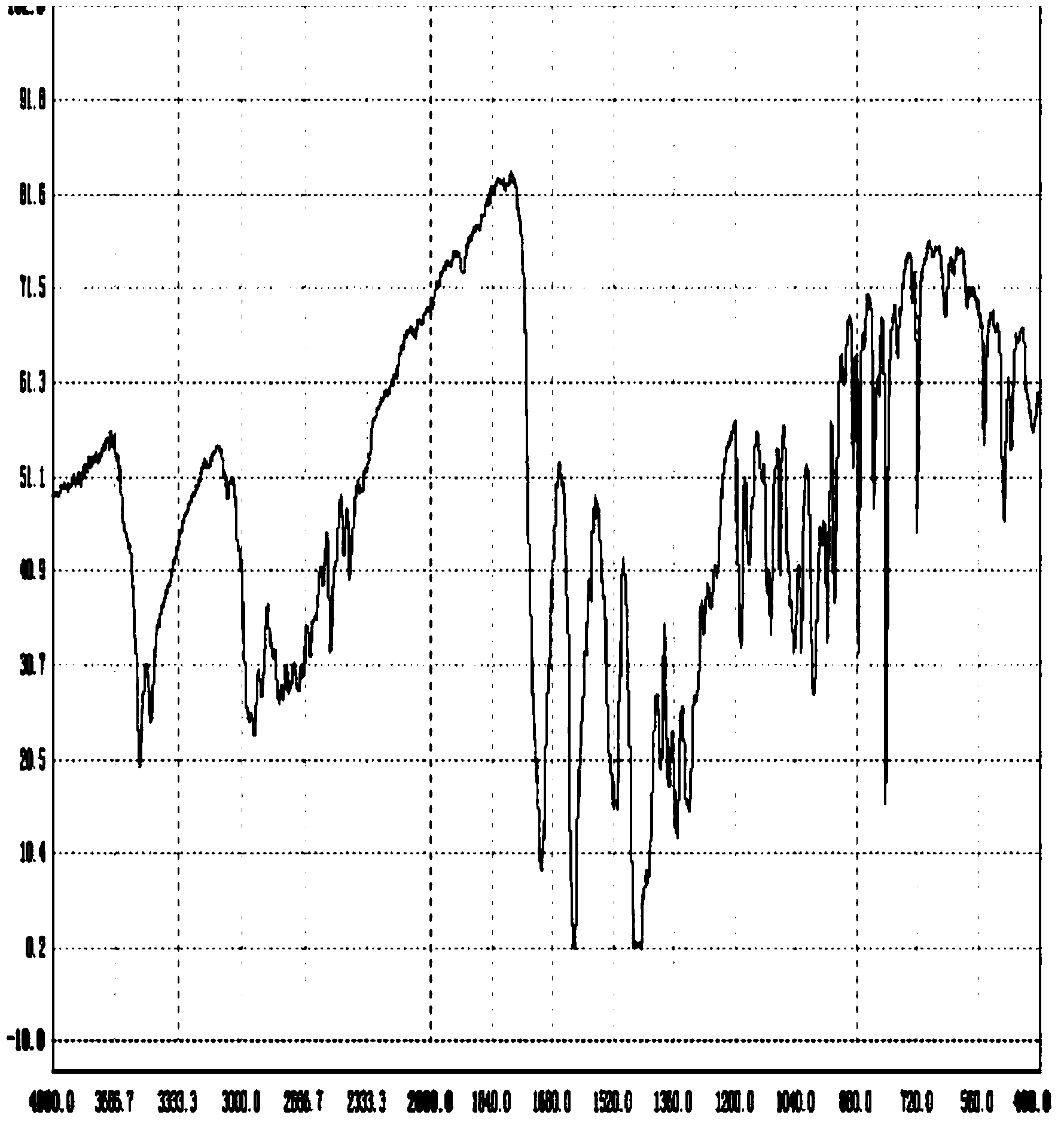
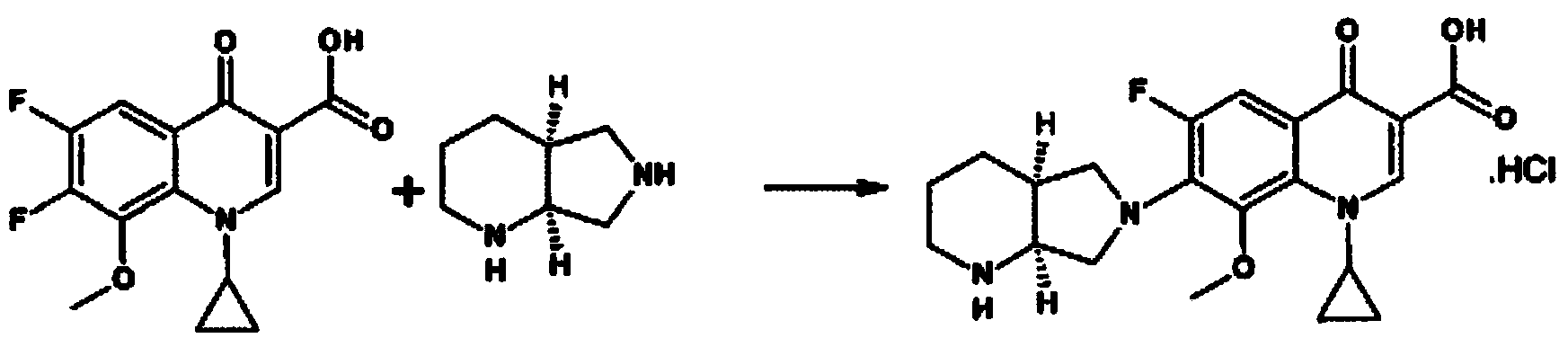
Novel preparation method of moxifloxacin hydrochloride
A technology for moxifloxacin hydrochloride and salt formation, which is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc. Reaction steps, the effect of reducing the generation of impurities and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 main ring chelate
[0052]Put 240g of acetic anhydride into a small reactor and heat to 70°C, slowly add 36g of boric acid between 70-90°C, heat up and reflux for 1 hour, then cool to 70°C, add 120g of 1-cyclopropyl-6,7-difluoro under stirring -Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 480ml of ice water slowly , then add 480ml of cold water at 0-5°C, keep it at 0-5°C for 2h, the product precipitates, filters, washes with 480ml of water, and vacuum-dries at 40°C until the water content is 2.1%, to obtain 156.6g of the main ring chelate.
Embodiment 2
[0053] The preparation of embodiment 2 main ring chelates
[0054] Put 120g of acetic anhydride into the reaction flask and heat to 70°C, slowly add 12g of boric acid between 70-90°C, heat up and reflux for 1h, then cool to 70°C, add 30g of 1-cyclopropyl-6,7-difluoro- Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 210ml of ice water slowly, Then add 210ml of cold water at 0-5°C, keep it at 0-5°C for 2 hours, the product precipitates, filters, washes with 210ml of water, and vacuum-dries at 50°C until the water content is 2.6%, to obtain 38.2g of the main ring chelate.
Embodiment 3
[0055] The preparation of embodiment 3 main ring chelates
[0056] Put 100g of acetic anhydride into the reaction flask and heat to 70°C, slowly add 10g of boric acid between 70-90°C, heat up and reflux for 1h, then cool to 70°C, add 20g of 1-cyclopropyl-6,7-difluoro- Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 200ml of ice water slowly, Then add 200ml of cold water at 0-5°C, keep it at 0-5°C for 2 hours, the product precipitates, filters, washes with 200ml of water, and vacuum-dries at 50°C until the water content is 3.9%, to obtain 26.0g of the main ring chelate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com