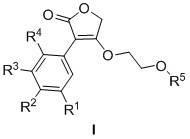
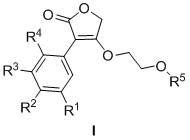
3-aryl-4-(2-acyloxy ethyoxyl)-2(5H)-furanone compound and preparation method and application thereof
A technology of acyloxyethoxy and furanone, applied in the application field of preparing antibacterial drugs, can solve the problems of short life cycle, reduced effectiveness and the like, and achieve the effects of high bacteriostatic activity, good inhibition and killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
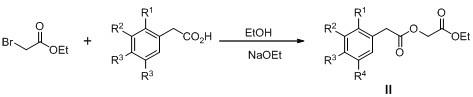
[0028] Step 1: Dissolve 2.7g (0.04mol) EtONa in 50mL of absolute ethanol, then add 6.45g (0.030mol) p-bromophenylacetic acid, add 5.1mL (0.045mol) ethyl bromoacetate after dissolving, heat up to 40 Between ~50°C, react for 10 hours, add 70 mL of water, extract three times with 200 mL of AcOEt, wash with saturated brine until neutral, dry, concentrate, and purify by silica gel (200-300 mesh) column chromatography (AcOEt:petroleum ether=1:6) , to obtain 7.7 g of ethyl p-bromophenylacetoxyacetate as a colorless oily product, with a yield of 85%.
[0029] Step 2: Take 7.2g (0.024mol) of ethyl p-bromophenylacetoxyacetate, dissolve it in 60mL of anhydrous THF, add 0.57gNaH (0.025mol), stir at room temperature for 5h, after the reaction is complete, add 100mL of water, and use 240mL diethyl ether was extracted three times, the aqueous layer was acidified with 5M hydrochloric acid, and a precipitate was precipitated, left to stand, filtered, washed, and dried to obtain a pale yellow s...
Embodiment 3
[0040] Bacteria were suspended in MH medium at a concentration of approximately 10 5 cfu mL -1 , add the bacterial solution to a 96-well plate (100 μL of bacterial solution per well), use the culture medium as a blank control, use DMSO instead of a test substance as a negative control, use penicillin G as a positive control for Gram-positive bacteria, and use gram-positive bacteria as a positive control. Kanamycin was used as a positive control for Lambert-negative bacteria, and ketoconazole was used as a positive control for fungi. Dissolve the test substance in DMSO to prepare 1600, 800, 400, 200, 100, 50 μg·mL -1 solution (for MIC 50 Less than 5μg·mL -1 Yes, when carrying out one-step experiment, the prepared concentration gradient is 100, 50, 25, 12.5, 6.25 μg·mL -1 ), added to a 96-well plate at an amount of 11 μL per well [the final concentrations of the drug solution were 160, 80, 40, 20, 10, 5 μg·mL -1 (10, 5, 2.5, 1.25 and 0.63 μg·mL for the latter -1 )], four ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com