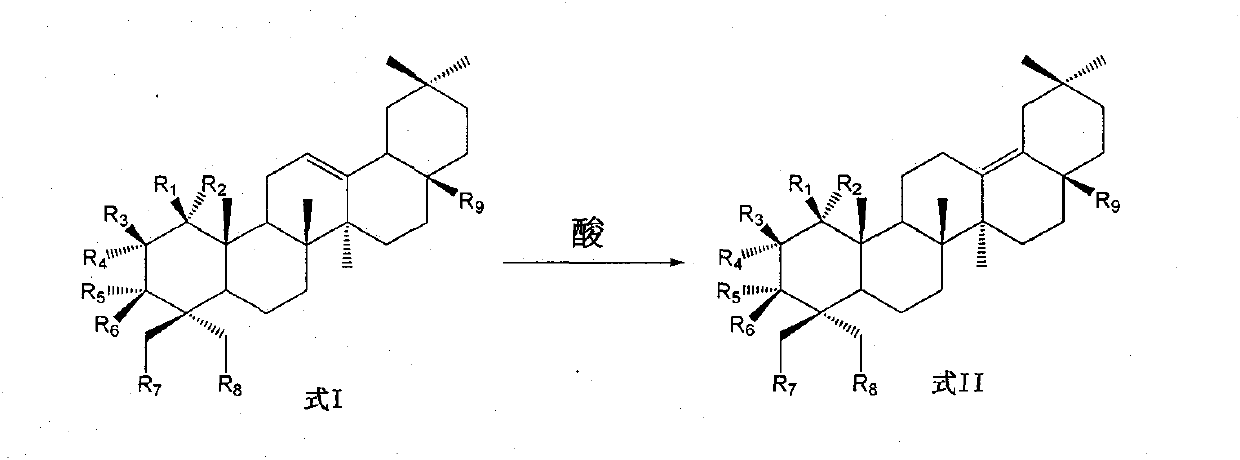
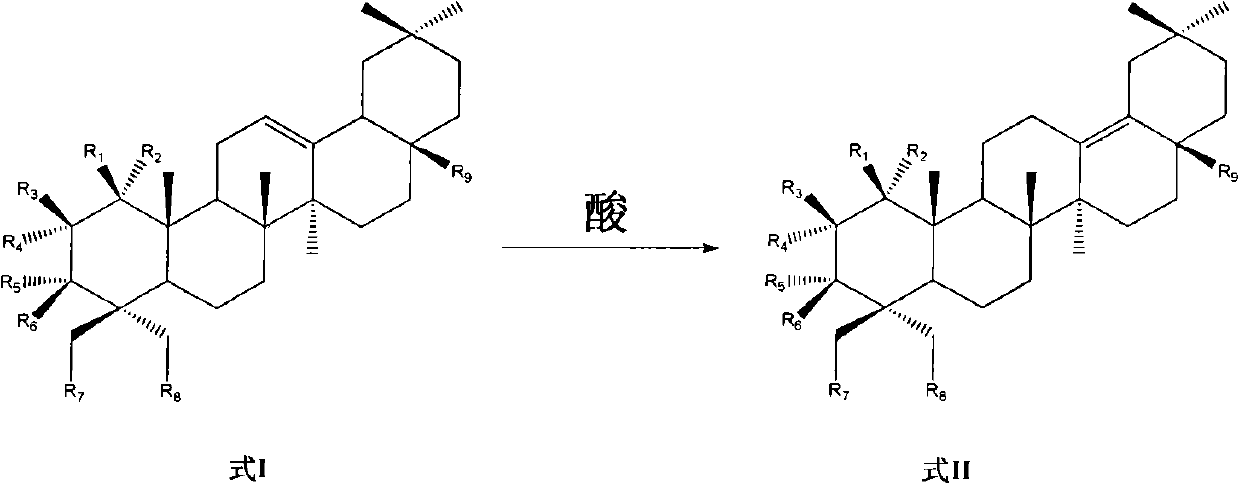
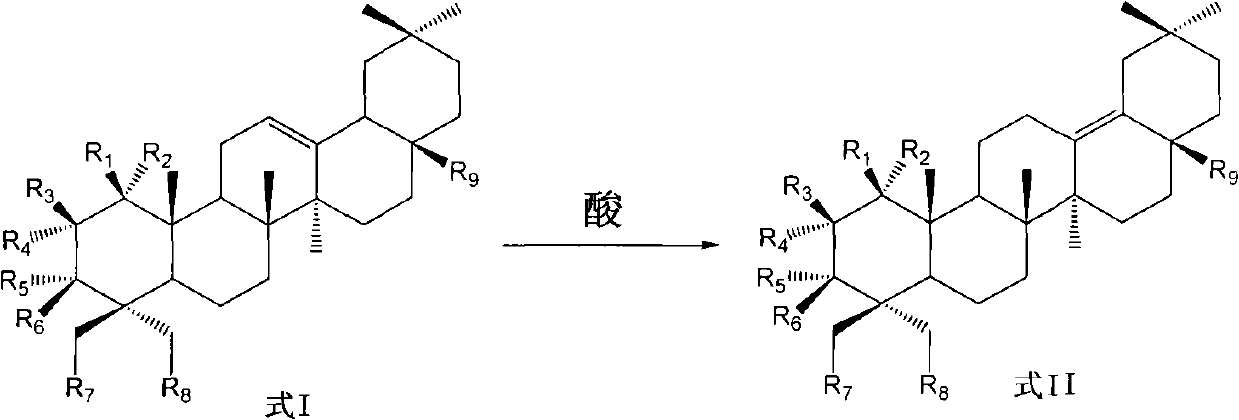
Preparation of 13 (18)-oleanane-type pentacyclic triterpene and derivatives of 13(18)-oleanane-type pentacyclic triterpene
A technology for oleanane and pentacyclic triterpenes, applied in the field of pharmacy, can solve problems such as limited plant sources, and achieve the effects of low price, little environmental pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 3β-hydroxy-13(18)-eneoleanolic acid-28-benzyl ester
[0038]
[0039] Add 8 grams of 3β-hydroxy-12-ene oleanolic acid-28-benzyl ester, 10 milliliters of trifluoroacetic acid, and 40 milliliters of dichloromethane to the reaction flask in sequence, and stir at room temperature for 4 hours. No obvious raw material residue is detected by TLC. , evaporated to remove the organic solvent, and obtained 3β-hydroxy-13(18)-ene oleanolic acid-28-benzyl ester by column chromatography.
[0040] 1 H NMR (CDCl 3 , 300M) δ0.74(3H, s), 0.78(3H, s), 0.81(3H, s), 0.87(3H, s), 0.88(3H, s), 0.99(3H, s), 1.15(3H , s), 0.72-1.95 (21H, m), 2.19-2.23 (1H, m), 2.42 (1H, d, J=14.2Hz), 2.73-2.77 (1H, m), 3.21-3.26 (1H, m ), 5.10 (1H, d, J = 12.4Hz), 5.16 (1H, d, J = 12.3Hz), 7.35 (5H, brs); 13 C NMR (CDCl 3 , 75M) δ15.5, 16.3, 17.5, 18.3, 21.0, 21.6, 24.2, 25.2, 27.0, 27.3, 28.1, 32.1, 32.6, 33.0, 34.9, 35.8, 36.8, 37.3, 38.8, 41.0, 41.3, 44.4, 48.5 , 50.7, 55.3, 66.2, 79.0...
Embodiment 2
[0042] Preparation of 3β-hydroxy-13(18)-eneoleanolic acid-28-benzyl ester
[0043]
[0044] Dissolve 1.5 ml of concentrated sulfuric acid in acetone, add 97 g of silica gel, stir at room temperature for 3 hours, evaporate the solvent, and obtain a solid acid in which sulfuric acid is adsorbed on silica gel. Add 1 g of 3β-hydroxy-12-ene oleanolic acid-28-benzyl in turn to the reaction flask, 1 g of the solid acid adsorbed on silica gel by the above-mentioned sulfuric acid, and 15 ml of dichloromethane, stir and react at room temperature for 8 hours, and detect by TLC When no obvious raw materials remained, the solid was filtered off, the organic solvent was distilled off, and 3β-hydroxy-13(18)-eneoleanolic acid-28-benzyl ester was obtained by column chromatography.
Embodiment 3
[0046] Preparation of 3β-hydroxy-13(18)-eneoleanolic acid-28-benzyl ester
[0047]
[0048] Add 0.2 g of 3β-hydroxy-12-ene oleanolic acid-28-benzyl ester, 0.26 g of montmorillonite KSF, CH 2 Cl 2 3 ml, heated and stirred to react for 12 hours, TLC detected that there was no obvious raw material residue, filtered with suction, and the filter cake was washed with CH 2 Cl 2 10 ml × 2 washes, and the filtrate was evaporated to dryness to obtain 3β-hydroxy-13(18)-ene oleanolic acid-28-benzyl ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com